All Solutions
Page 828: Assessment
$$
frac{E_{H}}{E_{U}}=frac{60times 25}{200}=7.5
$$
So one kilogram of hydrogen would give us 7.5 times more energy than one kilogram of uranium.
$^A_Ztextrm{X}$
where $Z=n_p$ and $A=n_p+n_n$.
$$
^{64}_{30}textrm{Zn}
$$
^{64}_{30}textrm{Zn}
$$
$$
M_d=M-Ztimes(m_p+m_e)-(A-Z)times m_n
$$
[M_d=M-16(m_p+m_e)-16m_n=31.97207-16times 1.007825-16times 1.008665]
[boxed{M_d=-0.29177textrm{ u}}]
$$
E_B=931.5times M_d=-931.5times 0.29177=boxed{-271.78textrm{ MeV}}
$$
$$
E_n=frac{E_B}{A}=-frac{271.78}{32}=boxed{-8.49textrm{ MeV}}
$$
textrm{a) }M_d=-0.29177textrm{ u}
$$
$$
textrm{b) }E_B=-271.78textrm{ MeV}
$$
$$
textrm{c) }E_n=-8.49textrm{ MeV}
$$
$$
M_d=M-Ztimes(m_p+m_e)-(A-Z)times m_n
$$
[M_d=M-7(m_p+m_e)-5m_n=12.0188-7times 1.007825-5times 1.008665]
[boxed{M_d=-0.0793textrm{ u}}]
$$
E_n=frac{931.5times M_d}{A}=-frac{931.5times 0.0793}{12}=boxed{-6.156textrm{ MeV}}
$$
$$
M_d=M-7(m_p+m_e)-5m_n=14.00307-7times 1.007825-7times 1.008665
$$
$$
M_d=-0.1123textrm{ u}
$$
$$
E_n=frac{931.5times M_d}{A}=-frac{931.5times 0.11236}{12}=boxed{-7.476textrm{ MeV}}
$$
so ti is harder to remove a nucleon from the latter isotope.
textrm{a) }E_n=-6.156textrm{ MeV}
$$
$$
textrm{b) }E_n=-7.476textrm{ MeV}
$$
$$
F=Kfrac{q_1q_2}{r^2}
$$
$$
F=9times 10^9timesfrac{1.6^2times 10^{-38}}{2times 10^{-15}}=boxed{58textrm{ N}}
$$
where we have used the fact that $q_1=q_2$.
F=58textrm{ N}
$$
$$
M_d=M-Ztimes n_p-(A-Z)times n_n
$$
Then the mass is given by the relation
$$
M=M_d+Ztimes n_p+(A-Z)times n_n
$$
$$
M_d=frac{E_b}{931.5textrm{ MeV}}
$$
so we can write the following
$$
M=-frac{28.3}{931.5}+2times 1.007825+2times 1.008665=boxed{4.0026textrm{ u}}
$$
M=4.0026textrm{ u}
$$
$$
^{A}_ZXrightarrow^{A-4}_{Z-2}Y+^4_2alpha
$$
$$
^{222}_{86}textrm{Rn}rightarrow^{218}_{84}textrm{Po}+^4_2alpha
$$
^{222}_{86}textrm{Rn}rightarrow^{218}_{84}textrm{Po}+^4_2alpha
$$
$$
^A_Ztextrm{X}rightarrow {^textrm{ A}_{(Z+1)}textrm{Y}}+^{textrm{ 0}}_textrm{-1}e+^0_0hatnu
$$
[^{89}_{36}textrm{Kr}rightarrow ^{89}_{37}textrm{Rb}+e^-+hatnu]
^{89}_{36}textrm{Kr}rightarrow ^{89}_{37}textrm{Rb}+e^-+hatnu
$$
textrm{a) }^{textrm{225}}_{textrm{ 89}}textrm{Ac}rightarrow^{textrm{4}}_{textrm{2}}textrm{He}+boxed{^{textrm{221}}_{textrm{ 87}}textrm{Fr}}
$$
textrm{b) }^{textrm{227}}_{textrm{ 88}}textrm{Ra}rightarrow^{textrm{ 0}}_{textrm{-1}}textrm{e}+boxed{^{textrm{227}}_{textrm{ 89}}textrm{Ac}+, ^{textrm{0}}_{textrm{0}}hatnu}
$$
textrm{c) }^{textrm{65}}_{textrm{29}}textrm{Cu}+, ^{textrm{1}}_{textrm{0}}textrm{n}rightarrowboxed{^{textrm{66}}_{textrm{29}}textrm{Cu}}rightarrow^{textrm{1}}_{textrm{1}}textrm{p}+boxed{^{textrm{65}}_{textrm{28}}textrm{Ni}}
$$
textrm{d) }^{textrm{235}}_{textrm{92}}textrm{U}+, ^{textrm{1}}_{textrm{0}}textrm{n}rightarrow ^{textrm{96}}_{textrm{40}}textrm{Zr}+3^{textrm{1}}_{textrm{0}}textrm{n}+boxed{^{textrm{137}}_{textrm{ 52}}textrm{Te}}
$$
textrm{a) }^{textrm{225}}_{textrm{ 89}}textrm{Ac}rightarrow^{textrm{4}}_{textrm{2}}textrm{He}+^{textrm{221}}_{textrm{ 87}}textrm{Fr}
$$
$$
textrm{b) }^{textrm{227}}_{textrm{ 88}}textrm{Ra}rightarrow^{textrm{ 0}}_{textrm{-1}}textrm{e}+^{textrm{227}}_{textrm{ 89}}textrm{Ac}+, ^{textrm{0}}_{textrm{0}}hatnu
$$
$$
textrm{c) }^{textrm{65}}_{textrm{29}}textrm{Cu}+, ^{textrm{1}}_{textrm{0}}textrm{n}rightarrow^{textrm{66}}_{textrm{29}}textrm{Cu}rightarrow^{textrm{1}}_{textrm{1}}textrm{p}+^{textrm{65}}_{textrm{28}}textrm{Ni}
$$
$$
textrm{d) }^{textrm{235}}_{textrm{92}}textrm{U}+, ^{textrm{1}}_{textrm{0}}textrm{n}rightarrow ^{textrm{96}}_{textrm{40}}textrm{Zr}+3^{textrm{1}}_{textrm{0}}textrm{n}+^{textrm{137}}_{textrm{ 52}}textrm{Te}
$$
$$
%=(frac{1}{2})^{frac{T}{T_{1/2}}}times 100%
$$
$$
%=(frac{1}{2})^{frac{6}{3}}times 100%=25%
$$
$$
%=(frac{1}{2})^{frac{9}{3}}times 100%=12.5%
$$
$$
%=(frac{1}{2})^{frac{12}{3}}times 100%=6.25%
$$
textrm{a) }%(textrm{6 days})=25%
$$
$$
textrm{b) }%(textrm{9 days})=12.5%
$$
$$
textrm{c) }%(textrm{12 days})=6.25%
$$
$$
A=(frac{1}{2})^{frac{T}{T_{1/2}}}A_0
$$
$$
frac{1}{8}A_0=(frac{1}{2})^{frac{T}{T_{1/2}}}A_0
$$
So we see that
$$
frac{T}{T_{1/2}}=3
$$
$T=3T_{1/2}=9$
So we should wait at least 9 days before entering the room again.
$$
^{textrm{11}}_{textrm{5}}textrm{B}+^{textrm{1}}_{textrm{1}}textrm{p}rightarrow{^{textrm{11}}_{textrm{6}}textrm{C}}+{^{textrm{1}}_{textrm{0}}textrm{n}}
$$
$$
^{textrm{11}}_{textrm{6}}textrm{C}rightarrow{^{textrm{11}}_{textrm{5}}textrm{B}}+{^{textrm{ 1}}_{textrm{+1}}textrm{e}}+{^{textrm{0}}_{textrm{0}}nu}
$$
textrm{a) Carbon}
$$
$$
textrm{b) }^{textrm{11}}_{textrm{5}}textrm{B}+^{textrm{1}}_{textrm{1}}textrm{p}rightarrow{^{textrm{11}}_{textrm{6}}textrm{C}}+{^{textrm{1}}_{textrm{0}}textrm{n}}
$$
$$
textrm{c) }^{textrm{11}}_{textrm{6}}textrm{C}rightarrow{^{textrm{11}}_{textrm{5}}textrm{B}}+{^{textrm{ 1}}_{textrm{+1}}textrm{e}}+{^{textrm{0}}_{textrm{0}}nu}
$$
$$
N=frac{E}{E_a}=frac{20times 5times 10^{12}}{3.21times 10^{-11}}=31.2times 10^{23} textrm{atoms}
$$
$$
n=frac{N}{N_A}=frac{31.2times 10^{23}}{6.022times 10^{23}}=5.17 textrm{mol}
$$
$$
m=ncdot M_{U_{235}}=5.17times 0.235=boxed{1.22textrm{ kg}}
$$
m=1.22textrm{ kg}
$$
$$
^textrm{2}_textrm{1}textrm{H}+^textrm{2}_textrm{1}textrm{H}rightarrow {^textrm{3}_textrm{2}textrm{He}}+{^textrm{A}_textrm{Z}textrm{X}}
$$
$$
Z=1+1-2=0
$$
$$
A=2+2-3=1
$$
so we have that the particle is a neutron, $^textrm{1}_textrm{0}textrm{n}$
$$
m=m_0cdot (frac{1}{2})^{frac{T}{T_{1/2}}}
$$
From which we can express the time T in several simple steps
$$
frac{m_0}{m}= 2^{frac{T}{T_{1/2}}}
$$
$$
logfrac{m_0}{m}=frac{T}{T_{1/2}}log{2}
$$
$$
T=T_{1/2}frac{log{frac{m_0}{m}}}{log2}=100timesfrac{log{frac{100}{3.1}}}{log2}=boxed{501textrm{years}}
$$
T=501textrm{years}
$$
frac{2}{3}$e. Therefore, three such quarks would have a total charge given as
$$
Q=3q=3times frac{2}{3}textrm{e}=boxed{2textrm{e}}
$$
Q=2textrm{e}
$$
$$
Q=q_{up}+q_{anti-down}=frac{2}{3}e+frac{1}{3}e=boxed{+e}
$$
Q=+e
$$
[u+bar u=frac{2}{3}-frac{2}{3}=0]
[d+bar u=-frac{1}{3}e-frac{2}{3}e=-1e]
[d+bar d=-frac{1}{3}e+frac{1}{3}e=0]
b) -1e
c) 0
$$
Q=d+d+u=-frac{1}{3}-frac{1}{3}+frac{2}{3}=0
$$
$$
Q=bar u+bar u+bar d=-frac{2}{3}-frac{2}{3}+frac{1}{3}=-1
$$
b) $Q=-1$
$$
tau=frac{dpi}{v}=frac{2times 10^3times 3.14}{3times 10^8}=boxed{2.1times 10^{-5}textrm{ s}}
$$
$$
N_r=frac{Delta E}{E_r}=frac{(400-8.5)times 10^9}{2.5times 10^6}=boxed{157times 10^3textrm{revolutions}}
$$
$$
T=tau times N_r=2.1times 10^{-5}times 157times 10^3=boxed{3.29textrm{ s}}
$$
$$
v=frac{S}{T}
$$
$$
S=vT=3times 10^8times 3.29=boxed{1times 10^9 textrm{ m}}
$$
textrm{a) }tau=2.1times 10^{-5}textrm{ s}
$$
$$
textrm{b) }N_r=157times 10^3textrm{ revolutions}
$$
$$
textrm{c) }T=3.29textrm{ s}
$$
$$
textrm{d) }S=1times 10^9 textrm{ m}
$$
$$
^textrm{A}_textrm{Z}textrm{X}+{^textrm{4}_textrm{2}}alpharightarrow^textrm{A+4}_textrm{Z+2}textrm{Y}
$$
$$
^textrm{14}_textrm{7}textrm{N}+{^textrm{4}_textrm{2}}alpharightarrow^textrm{18}_textrm{9}textrm{F}
$$
$$
^textrm{27}_textrm{13}textrm{Al}+{^textrm{4}_textrm{2}}alpharightarrow^textrm{31}_textrm{15}textrm{P}
$$
textrm{a) }^textrm{14}_textrm{7}textrm{N}+{^textrm{4}_textrm{2}}alpharightarrow^textrm{18}_textrm{9}textrm{F}
$$
$$
textrm{b) }^textrm{27}_textrm{13}textrm{Al}+{^textrm{4}_textrm{2}}alpharightarrow^textrm{31}_textrm{15}textrm{P}
$$
$$
m=m_0cdotfrac{1}{2^{frac{T}{T_{1/2}}}}
$$
$$
m=m_0cdotfrac{1}{2^{frac{60}{15}}}=boxed{frac{1}{16}m_0}
$$
m=frac{1}{16}m_0
$$
$$
^textrm{1}_textrm{1}textrm{p}+{^textrm{1}_textrm{0}textrm{n}}{rightarrow}{ ^textrm{2}_textrm{1}textrm{H}}
$$
$$
E=931.5textrm{MeV}cdotDelta M=931.5textrm{MeV}cdot(m_{D}-m_p-m_e)=931.5textrm{MeV}cdot(2.014102-1.007276-1.008665)
$$
$$
E=boxed{-1.71textrm{ MeV}}
$$
which is the energy obtained in this reaction (the final energy is lower than the initial one.)
E=-1.71textrm{ MeV}
$$
$$
K=Delta Mcdot 931.5=(232.0372-228.0287-4.0026)times 931.5=5.5 textrm{MeV}
$$
$$
K_{Th}=K-K_{alpha}=5.5-5.3=boxed{0.2textrm{ MeV}}
$$
K_{Th}=0.2textrm{ MeV}
$$
$$
p=frac{1}{2}frac{E}{c}=frac{1.02times 10^6times 1.6times 10^{-19}}{2times 3times 10^8}=boxed{272times 10^{-24}, frac{textrm{kg}cdottextrm{m}}{textrm{s}}}
$$
p=272times 10^{-24}, frac{textrm{kg}cdottextrm{m}}{textrm{s}}
$$
$$
N_F=frac{E}{E_F}=frac{4times 10^{26}}{25times 10^6times 1.6times 10^{-19}}=boxed{10^{38}, frac{textrm{fusions}}{textrm{s}}}
$$
N_F=10^{38}, frac{textrm{fusions}}{textrm{s}}
$$
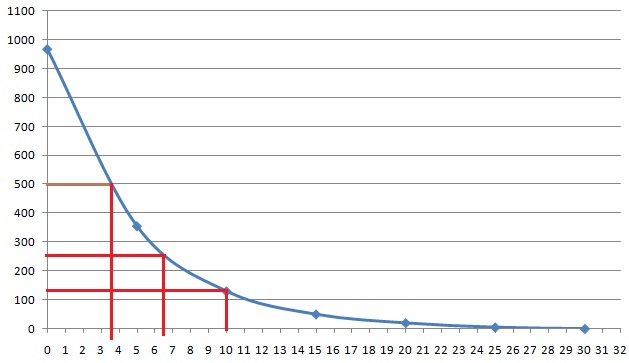
– $mathrm{Count}=[987,375,150,70,40,25,18]$
– $mathrm{Time~[min]}=[0,5,10,15,20,25,30]$
**Objective**
Determine the half-life od the isotope.
The half-life of an isotope $T_{1/2}$ is defined as the time in which the number of that isotope in the bulk $N$ decays to the half of the initial number $dfrac{N}{2}$.
Given data is the number of isotopes (Count) at a certain time. By plotting this data and fitting a line, we will be able to read the time it took for isotopes to decay to half of the initial value.
This means that the isotope, in the data we are given, is not alone. Every measurement contains cosmic rays too.
$$begin{align}
mathrm{Count-20}=[967,355,130,50,20,5,-2]
end{align}$$
This can be taken to be the count data due to isotope only.
Now we can plot data ($mathrm{Count-20}$, $mathrm{Time}$).

So it took approximately $4mathrm{~min}$ to get to the half of initial value of counts. Thus half-life $T_{1/2}$ is around $boxed{4mathrm{~minutes}}$.
$$
F=qvB=1.6times 10^{-19}times 1.7times 10^6times 0.91=boxed{2.48times 10^{-13}textrm{ N}}
$$
F=2.48times 10^{-13}textrm{ N}
$$
$$
B=frac{EMF}{lv}=frac{2times 10^{-3}}{0.1times 4}=boxed{5times 10^{-3}textrm{ T}}
$$
B=5times 10^{-3}textrm{ T}
$$
$$
lambda=frac{h}{mv}
$$
to express the velocity as:
v=frac{h}{mlambda}=frac{6.63times 10^{-34}}{9.11times 10^{-31}times 400times 10^{-9}}=boxed{1.82times 10^{3}frac{textrm{m}}{textrm{s}}}
$$
[E=frac{hc}{lambda}]
which after plugging the values and taking that $hc=1240times 10^{-9}$eV$cdot$m becomes
E=frac{1240times 10^{-9}}{400times 10^{-9}}
$$
$$
E=boxed{3.1textrm{ eV}}
$$
textrm{a) }v=1.82times 10^{3}frac{textrm{m}}{textrm{s}}
$$
$$
textrm{b) }E=3.1textrm{ eV}
$$
$$
K=E_{ph}-E_{ion}=14-13.6=boxed{0.4textrm{ eV}}
$$
K=0.4textrm{ eV}
$$
$$
V_R=V_b-V_d=6.67-0.7=boxed{5.97textrm{ V}}
$$
$$
R=frac{V_R}{I_R}=frac{5.97}{137times 10^{-3}}=boxed{43.6, Omega}
$$
V_R=5.97textrm{ V}
$$
$$
R=43.6,Omega
$$

