All Solutions
Page 770: Assessment
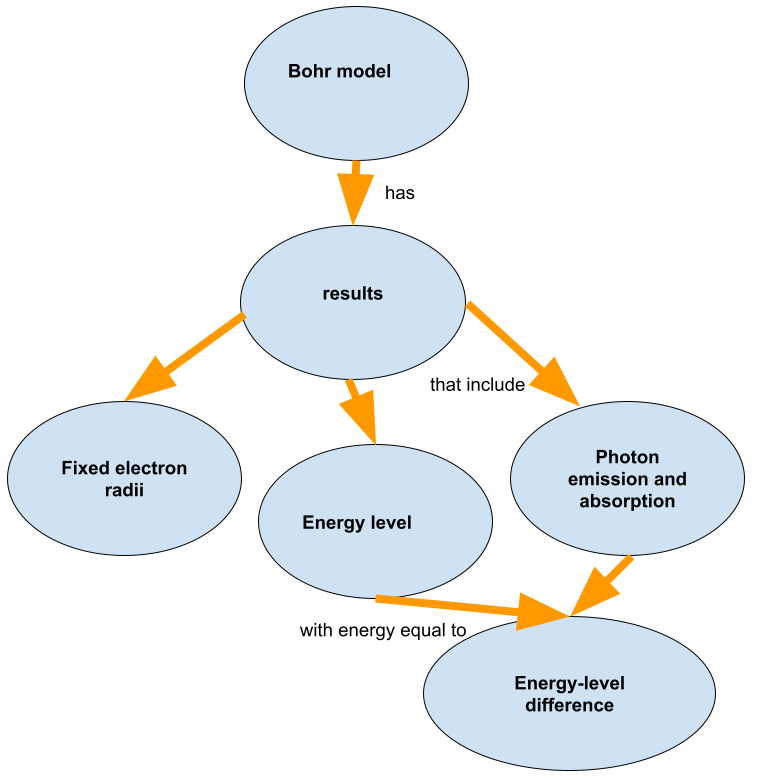
The second assumption was that change in energy happens in discrete jumps between energy levels and it is accompanied by absorption or emission of photons.
The third assumption was that angular momentum is quantized, too.
$textbf{Microwave Amplification by Stimulated Emission of Radiation}$
The water, in its macroscopic form is not a good example of quantization because it seems like a continuous quantity which can take any value.
$$
E_4-E_1=-4.95+10.38=5.43textrm{eV}
$$
and to get to $E_5$ is
$$
E_5-E_1=-3.71+10.38=6.67textrm{eV}
$$
and we need the photon energy to match exactly the transition energy for the excitation to happen.
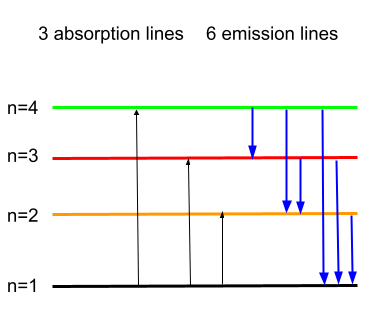
The complete emission and absorption spectrum is given below.
$$
E_i-E_{ph}=E_f
$$
$$
E_{ph}=E_i-E_f=5.16-2.93=2.23textrm{ eV}
$$
$$
E_{ph}=frac{hc}{lambda}
$$
where we can express $lambda$ as
$$
lambda=frac{hc}{E_{ph}}
$$
where $hc=1240times 10^{-9}$eV$cdot$m.
$$
lambda=frac{1240times 10^{-9}}{2.23}=boxed{556times 10^{-9}textrm{ m}}
$$
lambda=556times 10^{-9}textrm{ m}
$$
$$
E_{m1}=E_{21}+E_{ph}=2.93+1.2=4.13textrm{ eV}
$$
$$
E_6-E_{ph}=E_2
$$
where we read the $E_6$ and $E_2$ values from the figure 28-22.
$$
E_{ph}=E_6-E_2=5.16-2.93=boxed{2.23textrm{ eV}}
$$
E_{ph}=2.23textrm{ eV}
$$
$$
E_{6ion}=E_{ion}-E_{6}=6.08-5.16=0.92textrm{ eV}
$$
$$
E_{ph}=frac{hc}{lambda}=frac{1240times 10^{-9}}{600times 10^{-9}}=2.07textrm{ eV}
$$
$$
K_{e}=E_{ph}-E_{6ion}=2.07-0.92=boxed{1.15textrm{ eV}}
$$
K_{e}=1.15textrm{ eV}
$$
$$
E_{n}=-frac{13.6}{n^2}
$$
[E_2=-frac{13.6}{2^2}=boxed{-3.4textrm{ eV}}]
[E_7=-frac{13.6}{7^2}=boxed{-0.278textrm{ eV}}]
E_2=-3.4textrm{ eV}
$$
$$
E_7=-0.278textrm{ eV}
$$
$$
E_{n}=-frac{13.6}{n^2}
$$
[E_2=-frac{13.6}{2^2}=-3.4textrm{ eV}]
[E_7=-frac{13.6}{7^2}=-0.278textrm{ eV}]
[E_{7rightarrow2}=E_7-E_2=-0.278+3.4=boxed{3.12textrm{ eV}}]
E_{7rightarrow2}=3.12textrm{ eV}
$$
$$
E_{6ion}=E_{ion}-E_{6}=10.38-7.7=boxed{2.68 textrm{ eV}}
$$
$$
E_6-E_{ph}=E_2
$$
$$
E_{ph}=E_6-E_2=7.7-4.64=boxed{3.06textrm{ eV}}
$$
textrm{a) }E_{6ion}=2.68textrm{ eV}
$$
$$
textrm{b) }E_{ph}=3.06textrm{ eV}
$$
$$
E_{ph}=E_5-E_4=-3.71+4.95=1.24textrm{ eV}
$$
$$
f=frac{E_{ph}}{h}=frac{1.24times 1.6times 10^{-19}}{6.63times times 10^{-34}}=boxed{3times 10^{14}textrm{ Hz}}
$$
f=3times 10^{14}textrm{ Hz}
$$
$$
E_{n}=-frac{13.6}{n^2}
$$
[E_2=-frac{13.6}{2^2}=boxed{-3.4textrm{ eV}}]
[E_3=-frac{13.6}{3^2}=boxed{-1.51textrm{ eV}}]
[E_4=-frac{13.6}{4^2}=boxed{-0.85textrm{ eV}}]
[E_5=-frac{13.6}{5^2}=boxed{-0.544textrm{ eV}}]
[E_6=-frac{13.6}{6^2}=boxed{-0.378textrm{ eV}}]
E_2=-3.4textrm{ eV}
$$
$$
E_3=-1.51textrm{ eV}
$$
$$
E_4=-0.85textrm{ eV}
$$
$$
E_5=-0.544textrm{ eV}
$$
$$
E_6=-0.378textrm{ eV}
$$
$E_{6rightarrow5}$
$E_{6rightarrow3}$
$E_{4rightarrow2}$
$$
E_{5rightarrow2}
$$
$$
E_{5rightarrow3}
$$
$$
E_{n}=-frac{13.6}{n^2}
$$
[E_2=-frac{13.6}{2^2}=-3.4textrm{ eV}]
[E_3=-frac{13.6}{3^2}=-1.51textrm{ eV}]
[E_4=-frac{13.6}{4^2}=-0.85textrm{ eV}]
[E_5=-frac{13.6}{5^2}=-0.544textrm{ eV}]
[E_6=-frac{13.6}{6^2}=-0.378textrm{ eV}]
[E_{6rightarrow5}=E_6-E_5=-0.378+0.544=boxed{0.166textrm{ eV}}]
[E_{6rightarrow3}=E_6-E_3=-0.378+1.51=boxed{1.13textrm{ eV}}]
[E_{4rightarrow2}=E_4-E_2=-0.85+3.4=boxed{2.55textrm{ eV}}]
[E_{5rightarrow2}=E_5-E_2=-0.544+3.4=boxed{2.86textrm{ eV}}]
[E_{5rightarrow3}=E_5-E_3=-0.544+1.51=boxed{0.97textrm{ eV}}]
textrm{a) }E_{6rightarrow5}=0.166textrm{ eV}
$$
$$
textrm{b) }E_{6rightarrow3}=1.13textrm{ eV}
$$
$$
textrm{c) }E_{4rightarrow2}=2.55textrm{ eV}
$$
$$
textrm{d) }E_{5rightarrow2}=2.86textrm{ eV}
$$
$$
textrm{e) }E_{5rightarrow3}=0.97textrm{ eV}
$$
$E_{6rightarrow5}$
$E_{6rightarrow3}$
$E_{4rightarrow2}$
$$
E_{5rightarrow2}
$$
$$
E_{5rightarrow3}
$$
$$
E_{n}=-frac{13.6}{n^2}
$$
[E_2=-frac{13.6}{2^2}=-3.4textrm{ eV}]
[E_3=-frac{13.6}{3^2}=-1.51textrm{ eV}]
[E_4=-frac{13.6}{4^2}=-0.85textrm{ eV}]
[E_5=-frac{13.6}{5^2}=-0.544textrm{ eV}]
[E_6=-frac{13.6}{6^2}=-0.378textrm{ eV}]
[E_{6rightarrow5}=E_6-E_5=-0.378+0.544=0.166textrm{ eV}]
[f_{6rightarrow5}=frac{E_{6rightarrow5}}{h}=frac{0.166times 1.6times 10^{-19}}{6.63times 10^{-34}}=boxed{4times 10^{13}textrm{ Hz}}]
[E_{6rightarrow3}=E_6-E_3=-0.378+1.51=1.13textrm{ eV}]
[f_{6rightarrow3}=frac{E_{6rightarrow3}}{h}=frac{1.13times 1.6times 10^{-19}}{6.63times 10^{-34}}=boxed{2.7times 10^{14}textrm{ Hz}}]
[E_{4rightarrow2}=E_4-E_2=-0.85+3.4=2.55textrm{ eV}]
[f_{4rightarrow2}=frac{E_{4rightarrow2}}{h}=frac{2.55times 1.6times 10^{-19}}{6.63times 10^{-34}}=boxed{6.2times 10^{14}textrm{ Hz}}]
[E_{5rightarrow2}=E_5-E_2=-0.544+3.4=2.86textrm{ eV}]
[f_{5rightarrow2}=frac{E_{5rightarrow2}}{h}=frac{2.86times 1.6times 10^{-19}}{6.63times 10^{-34}}=boxed{6.9times 10^{14}textrm{ Hz}}]
[E_{5rightarrow3}=E_5-E_3=-0.544+1.51=0.97textrm{ eV}]
[f_{5rightarrow3}=frac{E_{5rightarrow3}}{h}=frac{0.97times 1.6times 10^{-19}}{6.63times 10^{-34}}=boxed{2.3times 10^{14}textrm{ Hz}}]
textrm{a) }f_{6rightarrow2}=4times 10^{13}textrm{ Hz}
$$
$$
textrm{b) }f_{6rightarrow3}=2.7times 10^{14}textrm{ Hz}
$$
$$
textrm{c) }f_{4rightarrow2}=6.2times 10^{14}textrm{ Hz}
$$
$$
textrm{d) }f_{5rightarrow2}=6.9times 10^{14}textrm{ Hz}
$$
$$
textrm{e) }f_{5rightarrow3}=2.3times 10^{14}textrm{ Hz}
$$
$E_{6rightarrow5}$
$E_{6rightarrow3}$
$E_{4rightarrow2}$
$$
E_{5rightarrow2}
$$
$$
E_{5rightarrow3}
$$
by using their frequencies
$$
E_{n}=-frac{13.6}{n^2}
$$
[E_2=-frac{13.6}{2^2}=-3.4textrm{ eV}]
[E_3=-frac{13.6}{3^2}=-1.51textrm{ eV}]
[E_4=-frac{13.6}{4^2}=-0.85textrm{ eV}]
[E_5=-frac{13.6}{5^2}=-0.544textrm{ eV}]
[E_6=-frac{13.6}{6^2}=-0.378textrm{ eV}]
[textrm{a) }E_{6rightarrow5}=0.166textrm{ eV}]
[textrm{b) }E_{6rightarrow3}=1.13textrm{ eV}]
[textrm{c) }E_{4rightarrow2}=2.55textrm{ eV}]
[textrm{d) }E_{5rightarrow2}=2.86textrm{ eV}]
[textrm{e) }E_{5rightarrow3}=0.97textrm{ eV}]
$$
textrm{a) }f_{6rightarrow2}=frac{E}{h}=4times 10^{13}textrm{ Hz}
$$
$$
textrm{b) }f_{6rightarrow3}=frac{E}{h}=2.7times 10^{14}textrm{ Hz}
$$
$$
textrm{c) }f_{4rightarrow2}=frac{E}{h}=6.2times 10^{14}textrm{ Hz}
$$
$$
textrm{d) }f_{5rightarrow2}=frac{E}{h}=6.9times 10^{14}textrm{ Hz}
$$
$$
textrm{e) }f_{5rightarrow3}=frac{E}{h}=2.3times 10^{14}textrm{ Hz}
$$
$$
c=lambda f
$$
to express the wavelength
$$
lambda=frac{c}{f}
$$
[lambda_{6rightarrow2}=frac{c}{f_{6rightarrow2}}=frac{3times 10^8}{4times 10^{13}}=boxed{7500times 10^{-9}textrm{ m}}]
[lambda_{6rightarrow3}=frac{c}{f_{6rightarrow3}}=frac{3times 10^8}{2.7times 10^{14}}=boxed{1111times 10^{-9}textrm{ m}}]
[lambda_{4rightarrow2}=frac{c}{f_{4rightarrow2}}=frac{3times 10^8}{6.2times 10^{14}}=boxed{484times 10^{-9}textrm{ m}}]
[lambda_{5rightarrow2}=frac{c}{f_{5rightarrow2}}=frac{3times 10^8}{6.9times 10^{14}}=boxed{435times 10^{-9}textrm{ m}}]
[lambda_{5rightarrow3}=frac{c}{f_{5rightarrow3}}=frac{3times 10^8}{2.3times 10^{14}}=boxed{1304times 10^{-9}textrm{ m}}]
textrm{a) }lambda_{6rightarrow2}=7500times 10^{-9}textrm{ m}
$$
$$
textrm{b) }lambda_{6rightarrow3}=1111times 10^{-9}textrm{ m}
$$
$$
textrm{c) }lambda_{4rightarrow2}=484times 10^{-9}textrm{ m}
$$
$$
textrm{d) }lambda_{5rightarrow2}=435times 10^{-9}textrm{ m}
$$
$$
textrm{e) }lambda_{5rightarrow3}=1304times 10^{-9}textrm{ m}
$$
$$
Delta E=frac{hc}{lambda}=frac{1240times 10^{-9}}{94.3times 10^{-9}}=13.15textrm{ eV}
$$
$$
E_n-Delta E=E_1
$$
$$
E_{n}=E_1+Delta E=-13.6+13.15=-0.45textrm{ eV}
$$
$$
E_n=-frac{13.6}{n^2}=-0.45
$$
which can be solved for $n$ to have that
$$
n*2=frac{13.6}{0.45}=30.2
$$
So we see that $n=5.5$ and we are going to take that the level is $boxed{n=6}$.
$$
r=frac{hbar^2n^2}{Kmq^2}
$$
r=frac{1.055^2 times 10^{-68}times 3^2}{9times 10^9times 9.11times 10^{-31}times 1.6^2times 10^{-38}}=boxed{4.77times 10^{-10}textrm{ m}}
$$
$$
F=frac{1}{4pi varepsilon}frac{q^2}{r^2}
$$
F=frac{9times10^9times 1.6^2times 10^{-38}}{4.77^2times 10^{-20}}=boxed{1.01times 10^{-9}textrm{ N}}
$$
so we have that
$$
a=frac{F}{m}
$$
a=frac{1.01times 10^{-9}}{9.11times 10^{-31}}=boxed{1.11times 10^{21},frac{textrm{m}}{textrm{s}^2}}
$$
$$
a=frac{v^2}{r}
$$
so we have that
$$
v=sqrt{ar}
$$
v=sqrt{1.11times 10^{21}times 4.77times 10^{-10}}=boxed{7.28times 10^5,frac{textrm{m}}{textrm{s}}=frac{1}{412}c}
$$
textrm{a) }r=4.77times 10^{-10}textrm{ m}
$$
$$
textrm{b) }F=1.01times 10^{-9}textrm{ N}
$$
$$
textrm{c) }a=1.11times 10^{21},frac{textrm{m}}{textrm{s}^2}
$$
$$
textrm{d) }v=7.28times 10^5,frac{textrm{m}}{textrm{s}}=frac{1}{412}c
$$
$$
E=frac{hc}{lambda}
$$
where $hc=1240times 10^{-9}$eV$cdot$m.
[E=frac{1240times 10^{-9}}{840times 10^{-9}}=boxed{1.48textrm{ eV}}]
E=1.48textrm{ eV}
$$
$$
E=frac{hc}{lambda}
$$
and it can be used to express the wavelength as
$$
lambda=frac{hc}{E}
$$
where $hc=1240times 10^{-9}$.
[lambda=frac{1240times 10^{-9}}{2.9}=boxed{428times 10^{-9}textrm{ m}}]
and this wavelength belongs to the visible, blue part of the spectrum. \
lambda=428times 10^{-9}textrm{ m}
$$
$$
E=frac{hc}{lambda}
$$
where $hc=1240times 10^{-9}$eV$cdot$m.
[E=frac{1240times 10^{-9}}{10,600times 10^{-9}}=boxed{0.117textrm{ eV}}]
E=0.117textrm{ eV}
$$
$$
E=nfrac{hc}{lambda}
$$
so we have that
$$
frac{n_1}{lambda_1}=frac{n_2}{lambda_2}
$$
[frac{n_1}{n_2}=frac{lambda_1}{lambda_2}=frac{840}{427}=boxed{1.97}]
$$
P=dot nE_{ph}
$$
$$
dot n=frac{P}{E_{ph}}=frac{P}{frac{hc}{lambda}}=frac{lambda P}{hc}
$$
where $hc=1240times 10^{-9}$ eV$cdot$m.
[dot n=frac{5times 10^{-3}times 840times 10^{-9}}{6.63times 10^{-34}times 3times 10^8}=boxed{21times 10^{15}, frac{textrm{photons}}{textrm{s}}}]
textrm{a) }frac{n_1}{n_2}=1.97
$$
$$
textrm{b) }dot n=21times 10^{15}, frac{textrm{photons}}{textrm{s}}
$$
$$
E_{ph}=frac{hc}{lambda}
$$
where $hc=1240times 10^{-9}$eV$cdot$m.
E_{ph1}=frac{1240times 10^{-9}}{632.8times 10^{-9}}=boxed{1.96textrm{eV}}
$$
$$
E_{ph2}=frac{1240times 10^{-9}}{543.4times 10^{-9}}=boxed{2.28textrm{eV}}
$$
$$
E_{ph3}=frac{1240times 10^{-9}}{1152.3times 10^{-9}}=boxed{1.08textrm{eV}}
$$
b) red, green, infrared
$$
K=E_{ph}-E_{ion}
$$
[K=14-13.6=boxed{0.4textrm{ eV}}]
K=boxed{0.4textrm{ eV}}
$$
$$
r_n=frac{hbar^2n^2}{Kmq^2}
$$
[r_5=frac{1.055^2times 10^{-68}times 5^2}{9times 10^{9}times 9.11times 10^{-31}times 1.6^2times 10^{-38}}]
Finally,
[boxed{r_5=1.33times 10^{-9}textrm{ m}}]
[r_6=frac{1.055^2times 10^{-68}times 6^2}{9times 10^{9}times 9.11times 10^{-31}times 1.6^2times 10^{-38}}]
Finally,
[boxed{r_6=1.91times 10^{-9}textrm{ m}}]
r_5=1.33times 10^{-9}textrm{ m}
$$
$$
r_6=1.91times 10^{-9}textrm{ m}
$$
$$
E_n=-frac{13.6}{n^2}textrm{ eV}
$$
So the energy of the second energy level is
$$
E_2=-frac{13.6}{2^2}=-3.4textrm{ eV}
$$
and that makes that the ionization energy (zero level ) is
$$
E_{ion}=3.4textrm{ eV}
$$
$$
E_{ph}=frac{hc}{lambda}=frac{1240times 10^{-9}}{332times 10^{-9}}=3.73textrm{ eV}
$$
and since $E_{ph}>E_{ion}$ $boxed{textrm{the atom is ionized.}}$
$$
K=E_{ph}-E_{ion}=3.73-3.4=0.33textrm{ eV}
$$
[K=0.33times 1.6times 10^{-19}=boxed{0.53times 10^{-19} textrm{ J}}]
b) $K=0.53times 10^{-19} textrm{ J}$
$$
E_3=-frac{13.6}{3^2}=-1.51textrm{ eV}
$$
$$
E_{e}=E_3-E_1=-1.51+13.6=boxed{12.1textrm{ eV}}
$$
E=12.1textrm{ eV}
$$
$$
E_{12}=E_2-E_1=-3.4+13.6=10.2textrm { eV}
$$
$$
E_{ph}=frac{E_{12}}{2}=5.1 textrm{ eV}
$$
$$
lambda=frac{hc}{E_{ph}}
$$
lambda=frac{1240times 10^{-9}}{5.1}=boxed{243times 10^{-9}textrm{ m}}
$$
lambda=243times 10^{-9}textrm{ m}
$$
$$
E=frac{hc}{lambda}
$$
[Delta E_1=frac{1240}{579}=2.14 textrm{ eV}]
[Delta E_2=frac{1240}{546}=2.27 textrm{ eV}]
[Delta E_3=frac{1240}{436}=2.84 textrm{ eV}]
$$
boxed{Delta E_1=E_{8rightarrow5}}
$$
$$
boxed{Delta E_2=E_{6rightarrow4}}
$$
$$
boxed{Delta E_3=E_{6rightarrow3}}
$$
Delta E_1=E_{8rightarrow5}
$$
$$
Delta E_2=E_{6rightarrow4}
$$
$$
Delta E_3=E_{6rightarrow3}
$$
[Delta E_1=E_{8rightarrow5}]
[Delta E_2=E_{6rightarrow4}]
[Delta E_3=E_{6rightarrow3}]
so we see that the initial states in our problem are $E_5$, $E_4$ and $E_3$.
$$
E_{5rightarrow1}=6.67
$$
$$
E_{4rightarrow1}=4.86
$$
$$
E_{3rightarrow1}=4.64
$$
$$
E=frac{hc}{lambda}
$$
where we know that $hc=1240times 10^{-9}$ eV$cdot$m.
$$
lambda=frac{hc}{E}=frac{1240times 10^{-9}}{4.64}=boxed{267times 10^{-9}textrm{ m}}
$$
which is in the ultraviolet range and therefore invisible which is the final answer.
$$
r=frac{hbar^2n^2}{Kmq^2}
$$
so we see that the radius of the orbits is inversely proportional to the mass of the electron so if we reduce the mass by a factor of two the radius will increase by a factor of two.
$$
E_n=-frac{K^2q^4m}{2pihbar^2n^2}
$$
so we see that the energy of the orbits is proportional to the mass of the electron so if we reduce the mass by a factor of two the energy will decrease by a factor of two, too.
$$
lambda=frac{hc}{E}
$$
lambda=frac{1240times 10^{-9}}{5.1}=boxed{243textrm{nm}}
$$
textrm{Radii will be twice larger.}
$$
$$
textrm{Energies will be twice lower.}
$$
$$
lambda=243textrm{nm}
$$
Nevertheless, this model had some obvious flaws (violating the conservation of energy, not explaining the experimental data..) which were solved by Niels Bohr who introduced the stationary states, the first step towards the quantum mechanical model of the atom. His model managed to fit well all the experimental results concerning hydrogen and hydrogen-like models but that was its maximum.
converts the 808 nm light into a light with a wavelength of 1064 nm which is well in the infrared part of the spectra. Then, the 1064 nm light uses
a frequency doubling crystal that emits green light at a wavelength of 532nm as a result.
$$
F=qE
$$
So the electric field is expressed as
$$
E=frac{F}{q}
$$
E=frac{F}{q}=frac{0.027}{3times 10^{-7}}=boxed{9times 10^4, frac{textrm{N}}{textrm{C}}}
$$
E=9times 10^4, frac{textrm{N}}{textrm{C}}
$$
$$R_1,R_2,R_3,…,R_n$$
We want to connect these resistors in the circuit and find the total resistance $R_{total}$. Depending on how we put these resistors in the circuit we can have different outcomes for $R_{total}$.
$$begin{align}
R_{total~parallel}=dfrac{1}{R_1}+dfrac{1}{R_2}+dfrac{1}{R_3}+…+dfrac{1}{R_n}
end{align}$$
– Putting resistors in series gives total resistance as:
$$begin{align}
R_{total~series}=R_1+R_2+R_3+…+R_n
end{align}$$
$$begin{aligned}
R_{total}&=R_1+R_2+R_3+…+R_n
\&=R_0+R_0+R_0+R_0
\&=4cdot R_0
\&=4cdot 1mathrm{~Omega}
\&=4mathrm{~Omega} ~checkmark
end{aligned}$$
$$
EMF=BLv
$$
[EMF=5times 10^{-5}times 1 times 4 =boxed{0.2times 10^{-3} textrm{ V}}]
EMF=0.2times 10^{-3} textrm{V}
$$
$vB=E$
from which the magnetic field is expressed as
$$
B=frac{E}{v}
$$
[B=frac{1.4times 10^4}{2.8times 10^8}=boxed{50times10^{-6}textrm{ T}}]
B=50times10^{-6}textrm{ T}
$$
$$
qvB=frac{mv^2}{r}
$$
so we have that
$$
r=frac{mv}{qB}
$$
and in order to keep it constant the magnetic field would have to be increased, and in this case some 2000 times roughly.
$$
K_{max}=V_{stop}=7.3times 1.6times 10^{-19}=boxed{11.7times 10^{-19}textrm{ J}}
$$
K_{max}=11.7times 10^{-19}textrm{ J}
$$

