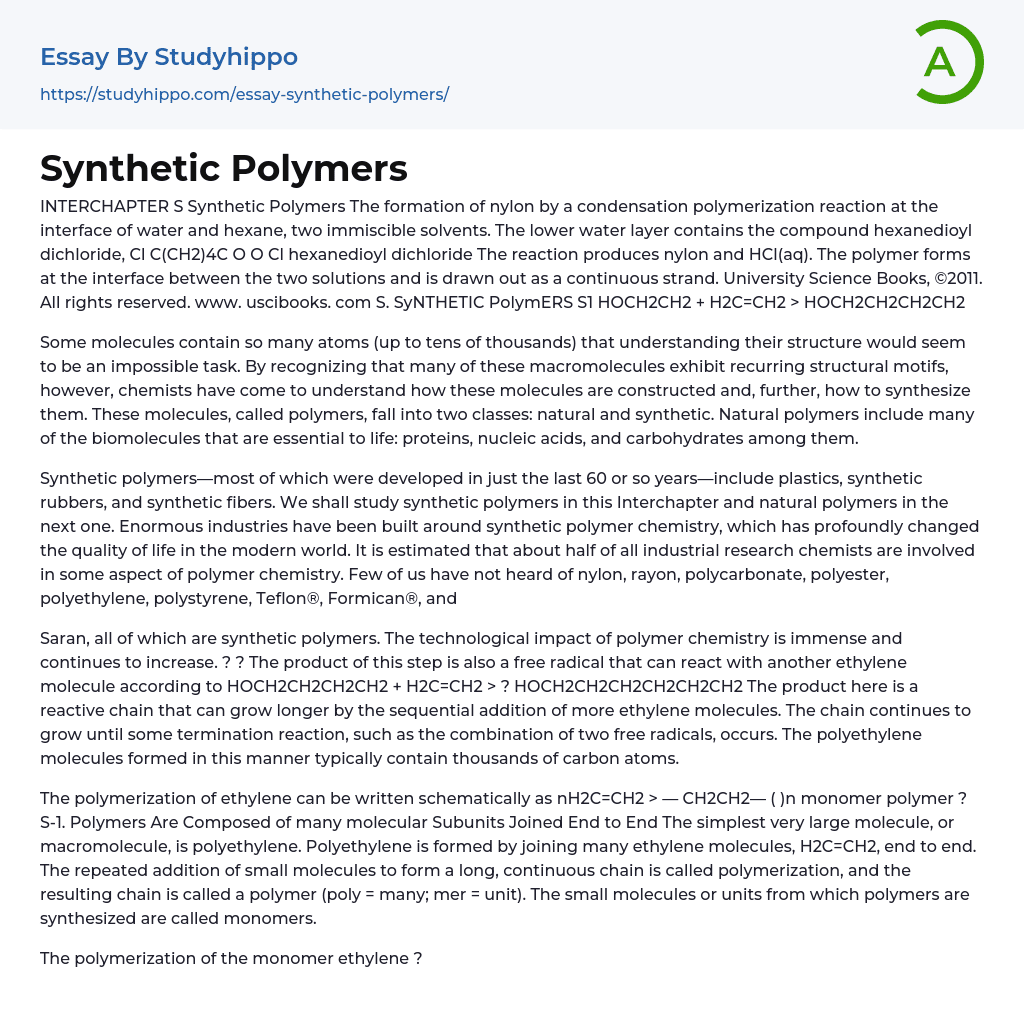
The formation of nylon by a condensation polymerization reaction at the interface of water and hexane, two immiscible solvents. The lower water layer contains the compound hexanedioyl dichloride, Cl C(CH2)4C O O Cl hexanedioyl dichloride The reaction produces nylon and HCl(aq). The polymer forms at the interface between the two solutions and is drawn out as a continuous strand.
Some molecules contain so many atoms (up to tens of thousands) that understanding their structure would seem to be an impossible task. By recognizing that many of these macromolecules exhibit recurring structural motifs, however, chemists have come to understand how these molecules are constructed and, further, how to synthesize them. These molecules, called polymers, fall into two classes: natural and synthetic. Natural polymers include many of the biomolecules that are essential to life: proteins, nucleic acids, and carb
...ohydrates among them.
Synthetic polymers—most of which were developed in just the last 60 or so years—include plastics, synthetic rubbers, and synthetic fibers. We shall study synthetic polymers in this Interchapter and natural polymers in the next one. Enormous industries have been built around synthetic polymer chemistry, which has profoundly changed the quality of life in the modern world. It is estimated that about half of all industrial research chemists are involved in some aspect of polymer chemistry.
Saran, all of which are synthetic polymers. The technological impact of polymer chemistry is immense and continues to increase. The product of this step is also a free radical that can react with another ethylene molecule according to HOCH2CH2CH2CH2 + H2C=CH2 >HOCH2CH2CH2CH2CH2CH2
The product here is a reactive chain that can grow longer by the
sequential addition of more ethylene molecules. The chain continues to grow until some termination reaction, such as the combination of two free radicals, occurs. The polyethylene molecules formed in this manner typically contain thousands of carbon atoms.
The polymerization of ethylene can be written schematically as nH2C=CH2 > — CH2CH2— n monomer polymer S-1. Polymers Are Composed of many molecular Subunits Joined End to End The simplest very large molecule, or macromolecule, is polyethylene. Polyethylene is formed by joining many ethylene molecules, H2C=CH2, end to end. The repeated addition of small molecules to form a long, continuous chain is called polymerization, and the resulting chain is called a polymer (poly = many; mer = unit). The small molecules or units from which polymers are synthesized are called monomers.
The polymerization of the monomer ethylene can be initiated by a free radical, such as HO , the hydroxyl radical. (Recall from Section 7-7 that a free radical is a species having one or more unpaired electrons. The first step in the polymerization of ethylene is the reaction described by The notation — CH2CH2— means that the group enclosed in the parentheses is repeated n times; it also serves to identify the monomer unit. The free radical that initiates the polymerization reaction is not indicated because n is large and thus the end group constitutes only a trivial fraction of the large polymer molecule.
The precise number of monomer molecules incorporated into a polymer molecule is not important for typically large values of n. Polymer syntheses generally produce polymer molecules with a range of n values. The polymer properties are described in terms of
the average value of n. It makes little difference whether a polyethylene molecule consists of 5000 or 5100 monomer units, for example. Polyethylene is a tough, flexible plastic that is used in the manufacture of packaging films and sheets, wire and cable insulation, ice cube trays, refrigerator dishes, squeeze bottles, bags for foods and clothes, trash bags, and many other articles.
The polymerization reaction of ethylene is an addition polymerization reaction because it involves the direct addition of monomer molecules. Another type of polymerization reaction is a condensation polymerization reaction. In such a reaction, a small molecule, such as water, is split out as each monomer is added to the polymer chain. The polymerization product is called a condensation polymer. The synthesis of nylon is an example of a condensation polymerization reaction.
Nylon is formed by the reaction of a diamino compound, such as 1,6-diaminohexane, H N H 1,6-diaminohexane A condensation polymeriza- CH2CH2CH2CH2CH2CH2 N H H and a dicarboxylic acid, such as hexanedioic acid (adipic acid), OH C O CH2CH2CH2CH2 C O OH hexanedioic acid (adipic acid) These two molecules can be linked by the reaction described by the equation shown in the box below. The product in this reaction is called a dimer (di = two; mer = unit). Observe that one end of the dimer is an amino group, –NH2, and the other end is a carboxyl group, –COOH. The dimer can grow by the reaction of its amino end with a dicarboxylic acid monomer or by the reaction of its carboxyl end with a diamine monomer.
Repetition of this process yields nylon (Frontispiece), whose general formula is tion reaction,
such as the synthesis of nylon, generally involves two different monomers that combine to form the basic repeating unit of the polymer. A polymer made from more than one type of monomer is known as a copolymer, whereas one made from just one type of monomer is called a monopolymer. Nylon was first synthesized by W. H. Carothers of the Dupont Chemical Company in 1931. His work was carried on by his colleague Paul J. Flory, who was awarded the 1974 Nobel Prize in Chemistry for his research on he dynamics and physical properties of polymers. Their research played a major role in launching the U. S. polymer chemical industry. Over one thousand metric tons of nylon fiber are produced annually in the United States.
It is used to make strong, long-wearing fibers that find extensive use in rugs as well as hosiery, sweaters, and other clothing. Nylon resembles silk in many of its properties but is cheaper to produce. This resemblance to silk is not at all coincidental. Silk is a protein, and, as we shall learn in Interchapter T, proteins are polymers in which the monomers are linked together by amide bonds as in nylon.
- Accounting essays
- Marketing essays
- Automation essays
- Business Cycle essays
- Business Model essays
- Business Operations essays
- Business Software essays
- Corporate Social Responsibility essays
- Infrastructure essays
- Logistics essays
- Manufacturing essays
- Multinational Corporation essays
- Richard Branson essays
- Small Business essays
- Cooperative essays
- Family Business essays
- Human Resource Management essays
- Sales essays
- Market essays
- Online Shopping essays
- Selling essays
- Strategy essays
- Management essays
- Franchising essays
- Quality Assurance essays
- Business Intelligence essays
- Corporation essays
- Stock essays
- Shopping Mall essays
- Harvard Business School essays
- Harvard university essays
- Trade Union essays
- Cooperation essays
- News Media essays
- Waste essays
- Andrew Carnegie essays
- Inventory essays
- Customer Relationship Management essays
- Structure essays
- Starting a Business essays
- Accounts Receivable essays
- Auditor's Report essays
- Balance Sheet essays
- Costs essays
- Financial Audit essays
- International Financial Reporting Standards essays
- Tax essays
- Accountability essays
- Cash essays
- Principal essays