Chapter 20: Nuclear Chemistry – Flashcards
Unlock all answers in this set
Unlock answersquestion
Nuclear chemistry
answer
chemistry of the nucleus rather than the electrons.
question
Chemical Reaction
answer
∙ does NOT change the nucleus ∙ different ISOTOPES behave essentially the SAME ∙rate is affected by temp, pressure, or catalyts ∙atoms in compounds behave differently than elements ∙relatively small energy change compared to nuclear reactions
question
Nuclear Reaction
answer
∙ CHANGES the nucleus ∙ different ISOTOPES often behave DIFFERENTLY ∙rate is UNAFFECTED by temp, pressure (within the range found on Earth), or catalyst ∙atom behaves the SAME whether it is elemental or in a compound ∙energy change can be millions of times greater than a chemical reaction
question
nucleons
answer
the nucleus is comprised of the two of these: protons and neutrons
question
atomic number
answer
number of protons, determines element
question
mass number
answer
number of protons and neutrons added together
question
isotopes
answer
∙NOT all atoms of the same element have the same mass. ∙They can have DIFFERENT numbers of NEUTRONS, and thus different MASS numbers. ∙Atoms that have the same atomic number but different mass numbers are isotopes of each other
question
radioactivity
answer
Spontaneous emission of particles or electromagnetic radiation from the nucleus The process is called *radioactive decay* or *nuclear decay.*
question
nuclear transmutation
answer
bombardment of nuclei by neutrons, protons, or other nuclei.
question
Nuclei decay into different nucleus:
answer
∙Alpha (a) decay: -->the nucleus loses TWO protons and TWO neutrons (i.e. helium nucleus) ∙Beta (b) decay: -->neutron→ proton + ejected electron ∙Gamma (g) emission: -->NO change in mass or atomic number ∙Positron emission (PE): -->proton→ neutron + ejected positron ∙electron capture (EC) --> proton + inner shell electron → neutron
question
The symbols for subatomic particles:
answer
∙ proton ¹₁H ¹₁p ∙neutron ¹₀n ∙electron ⁰₋₁e ⁰₋₁B ∙positron ⁰⁺₁e ⁰⁺₁B ∙alpha particle ⁴₂a ⁴₂He
question
nuclear reactions
answer
A nuclear equation is balanced when the number of nucleons and the sums of the charges are the same on both sides protons + neutrons→ protons→ 92 protons+ 146 neutrons 90 protons+ 144 neutrons 2 protons+ 2 neutrons
question
Nuclear Stability
answer
**neutron-to-proton ratio (n/p) --> There are more stable nuclei with 2, 8, 20, 50, 82, or 126 protons or neutrons -->More with even #'s -->All with atomic number > 83 are radioactive -->All isotopes of ₄₃Tc and ₆₁Pm are radioactive
question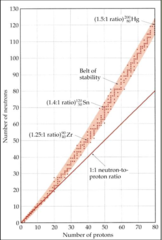
belt of stability

answer
∙Stable nuclei are located in this area of the graph ∙Most radioactive nuclei lie outside the belt. ∙Above the belt of stability, the nuclei have higher neutron-to-proton ratio.
question
Alpha Decay
answer
∙the loss of an a-particle (a helium nucleus). ⁴₂He or ⁴₂a *The nucleus loses two protons and two neutrons
question
Beta decay
answer
∙the loss of a B-particle (a high energy electron). no nucleons ↓ ⁰₋₁B or ⁰₋₁e
question
Beta emission
answer
*decomposition of a neutron to yield an ELECTRON and a PROTON. -->Because it is from decomposition of a **neutron**, the ELECTRON is coming from the *nucleus*, NOT from the orbitals. -->It is ejected from the nucleus. -->atomic number INCREASES by 1 ₀¹n → ¹₁p + ⁰₋₁e (n→ p +) *e⁻*)→→→
question
Gamma radiation
answer
∙the loss of a y-ray, which is made up of high-energy photons (electromagnetic radiation of very short wavelength). ∙Almost always accompanies the LOSS of a NUCLEAR particle, but is usually omitted from nuclear equations. ∙Emission of gamma rays causes NO change in mass or atomic number
question
Positron Emission
answer
∙involves the conversion of a proton in the nucleus into a neutron plus an ejected positron. proton → neutron + positron ∙atomic number DECREASES by 1 ¹₁p → ¹₀n + ⁰₁e (p→ n + (*E+*)→→
question
positron
answer
∙particle that has the same mass as but an opposite charge to that of an electron. ∙are very short-lived. When they collide with electrons, they are converted to gamma rays. positron + electron → 2 gamma rays →no nucleons⁰₁B or ⁰₁e
question
electron capture
answer
∙electron is captured from the inner shell of the surrounding electron cloud. (¹₁p) proton + (⁰₋₁) electron → (¹₀n) neutron *atomic number DECREASES by 1
question
Nuclear Stability
answer
∙Nuclei ABOVE the "belt of stability" have too many NEUTRONS. ∙They tend to decay by emitting *BETA particles.* ¹₀n → ¹₁p + ⁰₋₁e _______________________________ ∙Nuclei BELOW the belt have too many protons ∙They tend to become MORE STABLE by *positron emission* or *electron capture.* ¹₁p → ¹₀n + ⁰₁e or ¹₁p + ⁰₋₁e → ¹₀n ∙There are NO stable nuclei with an atomic number greater than 83 (no more "belt of stability"). i.e. All nuclei with more than 83 protons are radioactive. ∙Nuclei with such large atomic numbers (>83) tend to decay by *alpha emission.* ⁴₂He or ⁴₂a₂ ∙ Large nuclei emit large particles.
question
radioactive decay series
answer
is a sequence of nuclear reactions that ultimately result in the formation of a stable isotope.
question
parent isotope
answer
the beginning radioactive isotope
question
daughter isotope
answer
the product isotope
question
Einstein's famous equation expresses this:
answer
E = mc² where E = energy, m = mass, and c = the speed of light (2.9979 x 10⁸ m/s). **The law of conservation of energy = The law of conservation of mass
question
nuclear binding energy
answer
is the energy required to break up a nucleus into its component *protons and neutrons.*
question
nucleons (protons and neutrons)
answer
The mass of a nucleus is always LESS than the masses of the individual nucleons (protons and neutrons) it is composed of. =this is called=> *mass defect*
question
Loss in mass is converted to energy and can be quantified with Einstein's mass-energy equivalence relationship:
answer
ΔE = (Δm)c² →ΔE = energy of product - energy of reactant →Δm = mass of product - mass of reactant ∙(= mass of nucleus - sum of mass of nucleons) ∙(= mass lost when nucleus is formed)
question
Nuclear Binding Energy
answer
= -2.3686 x 10-11 J = energy lost (stability gained) when the nucleus of one atom is formed *the energy released when nucleons combine to form a nucleus; formation of the nucleus is energetically favorable *nuclear binding energy: the energy required to break up a nucleus into its component protons & neutrons
question
Binding Energy per Nucleon
answer
(Nuclear Binding Energy) / (number of nucleons) **highest BEPN is the most stable (requires the most energy per nucleon to breakup the nucleus) **nuclei w/ mass number ~40-100 have the highest Binding Energy per Nucleon, and thus are the most stable.
question
Kinetics (Rates) of Radioactive Decay
answer
∙Radioactive decay is a first-order process. ∙ The rate depends only on the number of radioactive nuclei N in the sample. Rate = kN where k is now called the *decay constant*,N is the number of *radioactive nuclei* in the sample
question
activity
answer
∙rate at which the sample decays -->expressed in units of *bequerels (Bq)*, which equals 1 nuclear disintegration per second. -->*curie (Ci) * is 3.7 x 1010 disintegrations/second.
question
Integrated rate law:
answer
ln Nt/N₀ = -kt where t= time interval of decay N₀= initial number of nuclei (at t=0) Nt= remaining number of nuclei after time t. -->Since mass and activity are each proportional to N, a *mass/mass ratio* or an *activity/activity ratio* can be used instead of N/N.
question
half-life, t½
answer
is the time for half of the material to decay. So for t½ , Nt / N₀ = ½ and thus: ** 0.693/k = t₁/₂
question
radiometric dating
answer
Because the half-life of a particular nuclide is constant, by: *comparing the % abundance of a radioactive isotope at a given point in time with the % abundance normally present,* one can find the age of an object. ∙Living systems take up ¹⁴C, but no longer do when they die. ∙By measuring the amount of ¹⁴C present compared to the natural abundance of ¹⁴C, an approximate age of a fossil can be determined.
question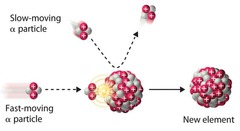
Nuclear transmutations

answer
nuclear reactions induced by colliding a neutron or another nucleus with the nuclide. ²⁷₁₃Al + ¹₀n→ ²⁴₁₁Na + ⁴₂He
question
Shorthand way of summarizing a nuclear transmutation:
answer
∙target nucleus (bombarding particle, ejected particle) product nucleus ²⁷₁₃Al (n,a) ²⁴₁₁Na ∙n is used for neutron, ∙p is used for proton, ∙a is used for alpha particle
question
Particle accelerators
answer
Charged particles must be moving at high speed to have enough energy to *overcome charge-charge repulsion* and *collide with the nucleus.* --> A particle accelerator is used.
question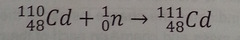
neutron capture
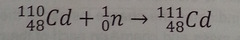
answer
Combination of a nucleus with a neutron takes place through this.
question
transuranium elements
answer
∙Elements above atomic number 92 (Uranium) ∙ because they follow Uranium on the periodic table. All of them are radioactive ∙can be made by bombardment of smaller nuclei with neutrons or other nuclei. e.g. bombardment with an accelerated a-particle (a charged particle of 2 protons and 2 neutrons):
question
Nuclear Fission and Fusion
answer
∙When small nuclei are *fused* into a larger nuclei of the same number of nucleons, *energy is released.* ∙ when large nuclei are fragmented *(fissioned)* into mid-sized nuclei with the same total number of nucleons, *energy is released.*
question
Nuclear fission
answer
∙a heavy nucleus (mass number > 200) divides to form smaller nuclei and one or more neutrons. ∙Heavy nuclei gain stability (release energy) when fragmented into mid-sized nuclei. --> Bombardment of the radioactive nuclide with a neutron starts the process.
question
nuclear chain reaction
answer
self-sustaining sequence of nuclear fission →Neutrons released in the transmutation strike other nuclei, causing their decay and the production of more neutrons.
question
critical mass
answer
minimum amount of fissionable material present for the chain reaction to be sustained
question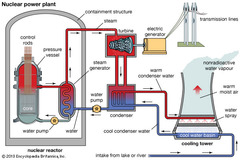
Nuclear Reactors

answer
In nuclear reactors the heat generated by the reaction is used to produce steam that turns a turbine connected to a generator
question
control rods
answer
capture some neutrons, keeping the system from reaching a dangerous supercritical mass
question
Light reactors
answer
use water as a coolant and uranium-235 as fuel, usually in the form of U₃O₈. The U-235 must be enriched to 3-4%.
question
Heavy reactors
answer
use D₂O, and the U-235 does not need to be enriched.
question
Breeder reactors
answer
add U-238, plutonium-239, or thorium-232 to the U-235. Each of these produces more fissionable material as it absorbs neutrons.
question
Nuclear Fusion
answer
Light nuclei are combined (fused) together to give heavier nuclei. -->Thermonuclear reactions: must be in the plasma state at several million kelvins
question
Hydrogen bomb
answer
Requires the energy from a fission detonation to initiate the following fusion reactions: ⁶₃Li +²₁H→ 2⁴₂a ²₁H +²₁H→ ³₁H + ¹₁H
question
tracers
answer
Use of tracers for diagnosis include: ∙Sodium-24: blood flow ∙Iodine-131: thyroid activity ∙Iodine-123: brain imaging ∙Technetium-99: organ imaging
question
Ionizing Radiation
answer
is radiation that removes an electron from an atom or molecule, making an ion. --> alpha, beta, gamma
question
Geiger counter
answer
radiation creates ions, which conduct a current
question
hydroxyl radical
answer
-->Ionization radiation in living tissue removes electrons from water. ∙OH, a neutral species with an unpaired electron. Radicals react with many things in a manner that generates more free radicals, so the resulting chain reaction can do a lot of damage in a biological system.
question
the rad (radiation absorbed dose).
answer
common unit for the absorbed dose of radiation 1 rad = 1 x 105 J/g of tissue irradiated
question
rem (roentgen equivalent for man)
answer
is determined from the number of rads: Number of rems = (number of rads) x (RBE) ∙RBE = the relative biological effectiveness. ∙A dose of 600 REMs will kill most humans



