Arterial Blood Gases (ABG)Lecture and Saunders Nursing 3 Test 5 – Flashcards
Unlock all answers in this set
Unlock answersquestion
What does arterial blood show
answer
Arterial blood shows the make-up of blood before it is distributed to the tissues
question
What 3 values do Arterial blood gas reflect
answer
The values reflect 1. Adequacy of Ventilation 2. Acid-base balance. 3. Arterial oxygenation and carbon dioxide levels
question
How to Obtain Arterial Blood
answer
1. Select a radial or femoral artery 2. Perform an Allen test if radial artery is selected 4. Use Heparinized syringe to draw the ABG 5. Label with: Time drawn/ FIO2 / O2 delivery rate and method / Ventilator settings /Patient temperature / Pulse oximetry O2 saturation 6. Place the sample on ice immediately 7. Promptly delivery it to the lab
question
Why and How do you perform an Allen test
answer
Radial artery requires Allen's test to test for adequate collateral blood flow from ulnar artery because of the potential for formation of an obstructing thrombus in the artery after puncture. Client elevates hand in a tight fist for 20 seconds with firm pressure held against radial and ulnar arteries. When patient opens hand it should blanche white. Examiner releases only ulnar compression and the hand is observed for "blushing". If the color of the hand does not return in 5-10 seconds the Allen test is considered positive and arterial puncture should not be attempted at that site
question
What are the 5 Values we test for with ABG's and what are their Normal ranges
answer
1. pH: 7.35 - 7.45 overall state 2. PCO2: 35 - 45 mm Hg (Acid or respiratory component) 3. HCO3: 22 - 26 mEq/L (Base or metabolic component) 4. PO2: 80 - 100 mmHg partial pressure of oxygen in arterial blood (***Not a pulse ox reading) 5. SaO2: 96 - 100% arterial O2 saturation
question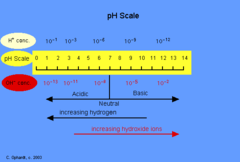
What is pH

answer
The concentration of H+
question
What is the normal pH for the human body
answer
The human body maintains a slightly alkaline pH of 7.35 - 7.45
question
Abnormal pH Values
answer
pH high = Alkalosis pH low = Acidosis
question
What processes work together to keep pH in a normal range
answer
Metabolic and respiratory processes work together to keep hydrogen (H+) levels in a normal range
question
What is PCO2
answer
The partial pressure of carbon dioxide dissolved in the arterial plasma It is the RESPIRATORY COMPONENT of acid-base regulation It measures the adequacy of alveolar ventilation (VA) Normal values range from 35-45
question
Abnormal PaCO2 Values
answer
Pa C02 high = Acidosis Pa C02 low =Alkalosis
question
HCO3
answer
The concentration of sodium bicarbonate in the blood Bicarbonate is a buffer and is the major form in which CO2 is transported to the lungs It is controlled by the kidneys. Normal range 22-26 mm hg
question
Abnormal HCO3 Values
answer
HCO3 high = Alkalosis HCO3 low = Acidosis
question
What are the regulators of Acids and Bases equilibrium in the body
answer
We control the amount of Buffers in our blood: 1. Respiratory system (Responses in minutes to hours) • Increased Respirations = Decreased CO2 • Decreased Respirations = Increased CO2 2. Renal system (Response in hours to days) The kidneys maintain homeostasis through excretion of waste products. •During Acidosis: • Reabsorbed HCO3 from tubular fluid • Secrete more H+ into collecting ducts to generate more bicarbonate; and more NH3 buffer is formed. •During Alkalosis: • Reabsorbed hydrogen ions •Secrete more HCO3 and NH3
question
What is a Buffer
answer
A chemical substance that resists changes in pH by accepting hydrogen ions from or donating hydrogen ions to solutions
question
What are 5 characteristics of Buffers and how do they help regulate Acids and Bases
answer
1. They are present in blood and tissues 2. They function only to keep the pH within the narrow limits of stability when too much acid or base is released into the system by absorbing the extra H+ ions or release H+ ions as needed 3. They serve as a transport mechanisms that carries excess hydrogen ions to the lungs 4. Once the buffers react they are consumed and leave the body ** Responds immediately to changes in hydrogen ion concentration in extracellular fluid (acid/base)
question
What is the primary buffer system in extracellular fluids
answer
Carbonic acid-bicarbonate system
question
Describe the Carbonic acid-bicarbonate system (Lungs)
answer
The system maintains a pH of 7.4 with a ratio of 20 parts HCO3 to 1 part H2CO3 (Carbonic acid). This 20 to1 ratio determines the H+ concentration of the body H2CO3 is controlled by the excretion of CO2 by the lungs Hco3 is controlled by the kidneys CO2 + H2O H2CO3 H+ + HCO3- Carbon dioxide + water dissociates to H+ and HCO3
question
What are the 4 Regulatory Systems for H+ in the blood
answer
1. Buffers Systems in Extracellular Fluid 2. Lungs 3. Kidneys 4. Potassium Exchange
question
What are the other buffer systems in extracellular fluids
answer
1. Hemoglobin System 2. Plasma Protein System 3. Phosphate Buffer System
question
Describe the Hemoglobin Buffer System (BLOOD)
answer
RBC contain Hgb Acid base balance maintained by CHLORIDE SHIFT HCO3 exits the RBC when Cl- enter HCO3 enters the RBC when Cl- exits
question
Describe the Plasma Protein Buffer System (Liver)
answer
The system works along with the liver to vary the H+ in the chemical structure of the plasma proteins The Plasma proteins will either attract or release H+
question
Describe the Phosphate Buffer System (Kidneys)
answer
System present in cells and body fluid Especially active in the Kidneys Acts like HCO3 and neutralizes excess H+
question
How does the Respiratory system help regulate Acids and Bases
answer
The Respiratory System Uses the Lungs to Eliminates CO2 (acid) A. ? resp. = ? CO2 out body ? CO2 in blood B. ? resp. = ? CO2 out body ? CO2 in blood ** Responds within minutes to hours to changes in acid/base
question
What is the functions of the lungs in acid base balance
answer
The lungs are the second defense of the body and interact with the buffer system to maintain acid base balance The actions of the lungs are reversible in controlling an excess or deficit Correction of a deficit can take 10 to 30 seconds ***The lungs can only inactivate H+ ions carried by carbonic acids. Hydrogen ions created by other mechanisms must be excreted by the kidneys
question
Explain the role of the lungs in Acidosis
answer
In Acidosis H+ ions are inactivated and exhaled: 1. As the pH decreases the respiratory rate and depth increases in an attempt to exhale acids. 2. Carbonic acid created by the neutralizing action of bicarbonate is carried to the lung and reduced to CO2 and water so it can be exhale
question
Explain the role of the lungs in Alkalosis
answer
In Alkalosis the pH increases and respiratory rate and depth decreases CO2 is retained and carbonic acid increases to neutralize and decrease the strength of excess bicarbonate
question
How does the Renal system help regulate Acids and Bases
answer
The Renal System 1.The Renal System Secretes hydrogen (H+) ions and reabsorbs bicarbonate (HCO3) ions 2. It also reabsorption and secretes electrolytes (e.g., Na, Cl) ** Responds occurs within hours to days to changes in acid/base
question
Explain the role of the renal system in acidosis
answer
In acidosis the pH decreases and excess H+ ions are secreted into the tubules and combined with buffers for excretion in the urine The excess H+ ions are excreted in the form of phosphoric acid The alterations of certain amino acids in the renal tubules results in a diffusion of ammonia into the kidneys; the ammonia combines with excess H+ ions and is excreted into the urine Bicarbonate is retained
question
Explain the role of the renal system in alkalosis
answer
In alkalosis the pH increases and excess bicarbonate ions move into the tubules, combine with sodium and are secreted in the urine
question
What is the functions of the kidneys in acid base balance
answer
The kidneys provide a more inclusive corrective response to acid base
question
What are the three names of an ABG Interpretation and what do they represent
answer
1. The First name indicates whether systemic compensation has maintained a normal pH A. Compensated B. Uncompensated 2. The Middle name represents the system that is causing the disturbance A. Respiratory B. Metabolic 3. The Last name reflects the type of disturbance A. Acidosis B. Alkalosis
question
What is hypoxemia
answer
A deficiency of oxygen in arterial blood as a result of any of the 3 findings below either found singly or in combination. 1. An abnormally low partial pressure of oxygen (mm Hg) 2. Content of oxygen (ml oxygen per dl blood) 3. Percent saturation of hemoglobin with oxygen
question
What is hypercapnia
answer
An increased of CO2 in the arterial blood (PaCO2), caused by hypoventilation of the alveoli, determined by minute volume This elevated CO2 results from hypoventilation as in: • COPD • CNS • Spinal cord damage • Tumors (In respiratory centers of brain) • Sleep apnea
question
What lab value is the Respiratory Function indicator
answer
PCO2
question
What is the relationship between the pH and PCO2 in respiratory imbalances
answer
There is an opposite relationship • The pH will be elevated with a decreased PCO2 (Alkalosis) • The pH will be decreased with an elevated PCO2 (Acidosis)
question
What lab value is the Metabolic Function indicator
answer
HCO3
question
What is the relationship between the pH and HCO2 in Metabolic imbalances
answer
There is a corresponding relationship • The pH and HCO3 will be elevated together (Alkalosis) • The pH and HCO3 will be decreased together (Acidosis)
question
Interpretation of ABGs requires answering what 5 questions
answer
1. Does the P02 show hypoxemia? 2. Does the pH show a disturbance? 3. Is the PC02 (respiratory system) causing the problem? 4. Is the HC03 (metabolic system) causing the problem? 5. Has compensation occurred?
question
Does the P02 show hypoxemia?
answer
Normal P02 is 80-100 mmHg Hypoxemia is a PO2 of < 80
question
What is the highest priority for a client who is hypoxic
answer
If the client's P02 is less than 80 mmHg, establishing an airway and oxygenation are the highest priority
question
Does the pH show a disturbance?
answer
The answer to this question tells you the first and last names of the ABG interpretation First name is: Compensated: if Normal pH (7.35-7.45) Uncompensated: if outside of normal pH range Last name if Uncompensated is: Acidosis: if pH 7.45 Last name if Compensated is: Acidosis: if pH Value is below 7.40 Alkalosis: if pH Value is above 7.40
question
Is the PC02 (respiratory system) causing the problem?
answer
Normal PC02 35-45 mmHg The middle name is Alkalotic: If PCO2 45
question
Is the HC03 (metabolic system) causing the problem?
answer
Normal HC03 is 22-26 mEq/l The middle name is Alkalotic: If HCO3 ; 26 mEq/L, The middle name is Acidotic: If HCO3 ; 22 mEq/L
question
How do we determine if we use PCO2 or HCO3 to determine the middle name.
answer
We must determine if CO2 or HCO3 matches the alteration. We use the mnemonic of ROME which stands for : Respiratory Opposite, Metabolic Equal The middle name is Respiratory if the pH and the PCO2 are going in opposite direction ? pH, ? PCO2 ? pH, ? PCO2 The middle name is Metabolic if the pH and the PCO2 are going in the same direction ? pH, ? HCO3 ? pH, ? HCO3
question
Has compensation occurred?
answer
In acidosis, the HC03 should rise to compensate In alkalosis, the PC02 should rise to maintain homeostasis Remember that the: Respiratory system: is very quick to react Renal System (kidneys): are slow and may take days to compensate
question
Four Types of Acid Base Disturbance

answer
Acidosis Respiratory PCO2 up HCO3 up Metabolic HCO3 down PCO2 down Alkalosis Respiratory PCO2 down HCO3 down Metabolic HCO3 up often none
question
What is the most common cause of acid-base imbalance
answer
Respiratory Acidosis
question
What is a Respiratory Acidosis
answer
An acid base disorder caused by primary defects in the function of the lungs or changes in normal respiratory patterns Acidosis is the retention of carbon dioxide in the blood, caused by any condition that impairs normal ventilation (decreased lung function). The CO2 creates carbonic acid (H2CO3) in the blood when it meets with the body's water.
question
When will we monitor for Respiratory Acidosis
answer
************************************************* Anytime a client has a condition that causes an obstruction of the airway or depresses the respiratory system,
question
What are the assessment findings of Respiratory Acidosis
answer
Blood Gas: 1. pH is decreased (pH = 45) (due to retaining C02 r/t ventilation depression) 3. HCO3 is normal or > with renal compensation Symptoms: 1. Vasodilatation 2. Cardiac dysrhythmias 3. Tachycardia 4. Somnolence 5. Decreased ventilation
question
What is the body's Compensation for Respiratory Acidosis
answer
**In an attempt to Compensation the Kidneys conserve bicarbonate (HCO3-) and secrete H+ into urine
question
Common causes of Respiratory Acidosis
answer
• Hypoventilation • Over sedation/anesthesia • Head Trauma • CNS Depressants • DRUG OVERDOSE • Diseases of the airways: Severe Asthma, Atelectasis, Bronchiectasis, COPD (Emphysema, chronic bronchitis), Pneumonia, Pulmonary Edema, Pulmonary Emboli (AABCPP • Neuromuscular diseases - ALS, diaphragm dysfunction and paralysis, Guillain-Barré syndrome, myasthenia gravis, muscular dystrophy, botulism.
question
Asthma
answer
Spasms resulting from allergens, irritants, or emotions cause the smooth muscles of the bronchioles to constrict, resulting in ineffective gas exchange
question
Atelectasis
answer
Excessive mucous collection, with the collapse of alveolar sacs caused by mucous plugs, infectious drainage, or anesthetic medications, resulting in ineffective gas exchange
question
Brain Trauma
answer
Excessive pressure on the respiratory center or medulla oblongata depresses respirations
question
Bronchiectasis
answer
Bronchi become dilated as a result of inflammation, and destructive changes and weakness in the walls of the bronchi occur
question
Bronchitis
answer
Inflammation causes airway obstruction, resulting in ineffective gas exchange
question
Central Nervous System
answer
Depressants such as opiods and anesthetics depress the respiratory center, leading to hypoventilation; CO2 is retained and H+ ion concentration increases
question
Emphysema
answer
Loss of elasticity of alveolar sacs restricts air flow in and out, primarily out, leading to an increased CO2 level
question
Hypoventilation
answer
CO2 is retained and the H+ ion concentration increases, leading to and acidotic stat; carbonic acid is retained and the pH decreases
question
Pulmonary edema
answer
Extracellular accumulation of fluid in pulmonary tissues causes disturbances in alveolar diffusion and perfusion
question
Pneumonia
answer
Excess mucous production and lung congestion causes airway obstruction, resulting in inadequate gas exchange
question
Pulmonary Emboli
answer
Emboli cause a pulmonary artery and airway obstruction, resulting in resulting in inadequate gas exchange
question
Neurological Clinical manifestations of Respiratory Acidosis
answer
Drowsiness Disorientation Dizziness Headache
question
Cardiovascular Clinical manifestations of Respiratory Acidosis
answer
Decreased Blood pressure Ventricular Fibrillation r/t hyperkalemia from compensation Warm, Flushed skin r/t peripheral vasodilation
question
Neuromuscular Clinical manifestations of Respiratory Acidosis
answer
Seizures
question
Respiratory Clinical manifestations of Respiratory Acidosis
answer
Hypoventilation with hypoxia because the lungs are unable to compensate when there is a respiratory problem
question
What are 11 common nursing interventions for Respiratory Acidosis
answer
1. Monitor for signs of Respiratory Distress 2. Administer O2 3. Semi Fowlers position 4. Turn, cough and deep breathe 5. Respiratory TX 6. Hydration to thin secretions 7. Suction, PRN 8. Improve ventilation instead of sedatives to reduce restlessness 9. Monitor K+ and other electrolytes 10. Administer antibiotics for infections 11. Prepare for endotracheal intubation and mechanical ventilation if CO2 increases above 50mm hg or signs of acute respiratory distress
question
verify What are the Clinical Manifestations of Metabolic Acidosis
answer
Hyperkalemia: 1. Shift of acid to ICF 2. Shift of K+ to the ECF Symptoms: 1. Anorexia 2. Nausea, Vomiting and Diarrhea 3. Warm, flushed skin 4. Cardiac dysrhythmias 5. CNS dysfunction 6. Headache 7. Tremors
question
What is Respiratory Alkalosis
answer
A deficit of carbonic acid and a decrease in H+ ion concentration that results from accumulation of base or From a loss of acid without a comparable loss of base in the body fluids Respiratory alkalosis is a disturbance in acid and base balance due to alveolar hyperventilation It causes: 1. A decreased PaCO2 (hypocapnia). 2. An increases the pH level (From increases in the ratio of bicarbonate concentration to PaCO2 )
question
What is Alveolar hyperventilation
answer
This hyperventilation develops when a strong respiratory stimulus causes the respiratory system to remove more carbon dioxide than is produced metabolically in the tissues
question
Who should we monitor for Respiratory Alkalosis
answer
***************************************************** Any client that has a condition that causes overstimulation of the respiratory system
question
What are the Assessment findings during Respiratory Alkalosis
answer
Initially the hyperventilation and respiratory stimulation cause abnormal rapid respirations (tachypnea) Symptoms: 1. Nausea 2. Vomiting 3. Tingling of fingers Blood Gas: 1. pH will be elevated (pH= ; 7.45) 2. PC02 will be decreased (PCO2= ; 35) (due to blowing off C02) 3. HCO3 will be normal or ; with renal compensation
question
What is the body's Compensation for Respiratory Alkalosis
answer
1. Lungs: Resp. rate and depth decrease to conserve CO2 2. Kidneys: excrete excess HCO3 into the urine
question
What are Common causes of Respiratory Alkalosis
answer
Results from conditions that cause overstimulation of the respiratory system 1. Hyperventilation 2. Pain 3. Anxiety and Hysteria 4. Severe anemia 5. Central nervous system lesions 6. Fever 7. Hypoxia 8. Overventilation by mechanical ventilators
question
Fever
answer
Causes increased metabolism, resulting in overstimulation of the respiratory system
question
Hyperventilation
answer
Rapid respirations cause the blowing off of CO2, leading to a decrease in carbonic acid
question
Hypoxia
answer
Stimulates the respiratory center in the brainstem, which causes an increase in the respiratory rate in order to increase O2; this causes hyperventilation which results in a decrease in CO2 levels
question
Hysteria
answer
Often neurogenic and related to psychoneurosis; however this condition leads to vigorous breathing and excessive exhaling of CO2
question
Over ventilation by mechanical ventilators
answer
The administration of O2 and the depletion of CO2 can occur from mechanical ventilation, causing the client to be hyperventilated
question
Pain
answer
Overstimulation of the respiratory center in the brainstem
question
Respiratory Alkalosis Neurological Clinical Manifestation
answer
Lethargy Lightheadedness Confusion
question
Respiratory Alkalosis Cardiovascular Clinical Manifestation
answer
Tachycardia Dysrhythmias r/t hypokalemia from compensating
question
Respiratory Alkalosis GI Clinical Manifestation
answer
Nausea Vomiting Epigastric Pain
question
Respiratory Alkalosis Neuromuscular Clinical Manifestation
answer
Tetany Numbness Tingling of extremities Hyperreflexia Seizures
question
Respiratory Alkalosis Respiratory Clinical Manifestation
answer
Hyperventilation because lungs are unable to compensate for respiratory problems
question
Nursing Interventions for Respiratory Alkalosis
answer
1. Monitor for s/s of Respiratory Distress 2. Provide emotional support and reassurance 3. Encourage appropriate breathing patterns and Assist with breathing techniques and aids 4. Provide cautious care with ventilator clients to prevent forced deep or rapid breaths 5. Monitor K+, Ca+ and other electrolyte levels 6. Give calcium gluconate if s/s of Tetany
question
What is Metabolic Acidosis
answer
Total concentration of buffer base that is lower than normal, with a relative increase in H+ ion concentration from the loss of too much base or the retention of too much acid
question
Who should we monitor for Metabolic Acidosis
answer
Monitor any client with sever diarrhea and diabetics with an insufficient supply of insulin
question
What are the Assessment findings during Metabolic Acidosis
answer
Blood Gas Findings: 1. pH will be decreased (pH = < 7.35) 2. PCO2 3. HC03 will be decreased (HCO3 = < 22) • The respiratory center in the brainstem is stimulated, and hyperventilation develops in an effort to compensate for the acidosis. Symptoms: (They are not specific.) Clients MAY report: • Varying degrees of dyspnea • Chest pain • Palpitations • Headache • Confusion • Generalized Weakness • Bone pain • Tachypnea • Tachycardia • Anorexia • Nausea **Fruity-smelling breath is a classic symptom of diabetic ketoacidosis (DKA). Children, also may present with nausea, vomiting, and decreased appetite
question
Some of the common causes of gain of base or loss of metabolic acids (metabolic alkalosis) are:a
answer
Gain of base: Increased ingestion of antacids or an excessive administration of sodium bicarbonate Loss of metabolic acids: vomiting, nasogastric suctioning, low potassium and/or chloride levels, diuretics, steroids, increase in aldosterone
question
Case Study 1: A client recovering from surgery in the post-anesthesia care unit (PACU) is difficult to arouse two hours following surgery. The nurse in the PACU has been administering Morphine Sulfate intravenously to the client for complaints of post-surgical pain. The client's respiratory rate is 7 per minute and demonstrates shallow breathing. The patient does not respond to any stimuli! The nurse assesses the ABCs (remember Airway, Breathing, Circulation!) and obtains ABGs STAT! Analyze the STAT results that have come back from the laboratory and show: pH = 7.15 Pa C02 = 68 mmHg HC03 = 22 mEq/L
answer
Uncompensated Respiratory Acidosis
question
Case Studies: Case Study 9 A young man is found at the scene of an automobile accident in a state of emotional distress. He tells the paramedics that he feels dizzy, tingling in his fingertips, and does not remember what happened to his car. Respiratory rate is rapid at 34/minute. Which primary acid-base disturbance is the young man at risk for if medical attention is not provided?
answer
Respiratory Alkalosis Client with Rapid Respiratory rate of 34 with clinical manifestations of dizzy and tingling in his fingertips
question
Case Studies: Case Study 3 A client, 5 days post-abdominal surgery, has a nasogastric tube. The nurse notes that the nasogastric tube (NGT) is draining a large amount (900 cc in 2hours) of coffee ground secretions. The client is not oriented to person, place, or time. The nurse contacts the attending physician and STAT ABGs are ordered. Analyze the STAT results that have come back from the laboratory and show: pH = 7.52 Pa C02 = 35 mmHg HC03 = 29 mEq/L
answer
Uncompensated Metabolic Alkalosis
question
Case Studies: Case Study 4 A client is admitted to the hospital and is being prepared for a craniotomy (brain surgery). The client is very anxious and scared of the impending surgery. He begins to hyperventilate and becomes very dizzy. The client looses consciousness and the STAT ABGs reveal: Analyze the STAT results that have come back from the laboratory and show: pH = 7.57 Pa C02 = 26 mmHg HC03 = 24 mEq/L
answer
Uncompensated Respiratory Alkalosis
question
Case Study 2: An infant, three weeks old, is admitted to the Emergency Room. The mother reports that the infant has been irritable, difficult to breastfeed and has had diarrhea for the past 4 days. The infant's respiratory rate is elevated and the fontanels are sunken. The Emergency Room physician orders ABGs after assessing the ABCs. Analyze the STAT results that have come back from the laboratory and show: pH = 7.37 Pa C02 = 29 mmHg HC03 = 17 mEq/L
answer
Compensated Metabolic Acidosis
question
What is the body's Compensation for Metabolic Acidosis
answer
Lungs: Hyperpnea with Kussmaul respiration to Increased CO2 exhaled by lungs (deep and rapid respirations)
question
What are 9 Common causes of Metabolic Acidosis
answer
Increased acids due to : 1. Renal failure, Acute Kidney Injury or CKD 2. Diabetic Ketoacidosis (DKA)/Alcoholic ketoacidosis 3. Aspirin Overdose 4. Anaerobic Metabolism (shock) 5. Loss of Base: Severe Diarrhea (We poop bases) 6. Insufficient Metabolism of Carbohydrates 7. Malnutrition / Starvation 8. High Fat Diet 9. Severe diarrhea (We poop base) **DKA: An insufficient supply of insulin in a client with
question
Diabetic mellitus/Diabetic or alcoholic ketoacidosis
answer
An insufficient supply of insulin causes increased fat metabolism, leading to an excess accumulation of ketones or other acids, the bicarbonate ends up being depleted
question
Case Studies: Case Study 5 A two-year-old is admitted to the hospital with a diagnosis of asthma and respiratory distress syndrome. The father of the infant reports to the nurse that he has observed slight tremors and behavioral changes in his child over the past three days. The attending physician orders routine ABGs following an assessment of the ABCs. The ABG results are: Analyze the STAT results that have come back from the laboratory and show: pH = 7.36 Pa C02 = 69 mmHg HC03 = 36 mEq/L
answer
Compensated Respiratory Acidosis
question
Salicylate ingestions
answer
Excessive ingestion can cause an increase in the H+ ion concentration
question
Case Studies: Case Study 6 A young woman, drinking beer at a party, falls and hits her head on the ground. A friend dials "911" because the young woman is unconscious, depressed ventilation (shallow and slow respirations), rapid heart rate, and is profusely bleeding from both ears. Which primary acid-base imbalance is this young woman at risk for if medical attention is not provided?
answer
Respiratory Acidosis secondary to depressed ventilations
question
High Fat Diet
answer
Causes a rapid accumulation of waste products of fat metabolism leading to a buildup of ketones and acid
question
Case Studies: Case Study 7 An 11-year old boy is admitted to the hospital with vomiting, nausea and overall weakness. The nurse notes the laboratory results: potassium: 2.9 mEq. Which primary acid-base imbalance is this boy at risk for if medical attention is not provided?
answer
Metabolic alkalosis Potassium normal range = 3.5-5.2 Client has low potassium of 2.9 mEq (decreased electrolytes) secondary to NV. So this is primarily a metabolic disorder.
question
Insufficient Carb metabolism
answer
When the O2 supply is not sufficient for the metabolism of carbs, lactic acid is produce and lactic acidosis results
question
Case Studies: Case Study 8 An elderly gentleman is seen in the emergency department at a community hospital. He admits to taking many tablets of aspirin (salicylates) over the last 24-hour period because of a severe headache. He complains of an inability to urinate. His vital signs are: Temp = 98.5; apical pulse = 92; respiration = 30 and deep. Which primary acid-base imbalance is the gentleman at risk for if medical attention is not provided?
answer
Metabolic Acidosis secondary to aspirin overdose
question
Malnutrition
answer
Improper metabolism of nutrients causes fat catabolism, leading to an excess build up of ketones and acids
question
Renal Insufficiency, Acute kidney injury or chronic kidney disease
answer
Increased waste products from protein metabolism are retained Acids increase and bicarbonate cant maintain acid base balance
question
Severe Diarrhea
answer
Intestinal and pancreatic secretions are normally alkaline, and the excess loss of base leads to acidosis
question
What is the nursing implications for Metabolic Acidosis
answer
1. Monitor for signs of respiratory distress 2. Assess LOC for CNS Depression 3. Monitor I;O's /provide fluid/electrolyte replacement 4. Administer IV solutions to increase base 5. Initiate Safety and Seizure precautions 6. Monitor K+ closely as metabolic acidosis resolves because K+ moves back into cells and levels decrease
question
What is Metabolic Alkalosis
answer
A condition with a deficit of carbonic acid and a decrease in H+ ion concentration that results from the accumulation of base or from a loss of acid without a comparative loss of base in the body fluids
question
What is the body's Compensation for Metabolic Alkalosis
answer
Compensatory mechanisms Decreased respiratory rate to increase plasma CO2
question
What are the Assessment findings during Metabolic Alkalosis
answer
Blood Gas Findings: 1. pH will be increased (pH= ; 7.45) 2. PCO2 normal or elevated in attempt to compensate 3. HC03 will be increased ( HCO3 = ; 26) Symptoms: 1. Cardiac dysrhythmias 2. Seizures 3. Confusion 4. Muscle twitching 5. Agitation
question
Common causes of Metabolic Alkalosis
answer
Prolonged vomiting (We vomit acid) Excessive Gastrointestinal suctioning Diurectic therapy Potassium deficit Hypochloremia
question
What is the nursing implications for potassium
answer
Monitor K+ levels closely in acid base imbalances because of K+ inverse shift with H+
question
What is the role of K+ in maintaining acid base balance
answer
Potassium moves across the cell membrane facilitated by transcellular shifts of H+ being drawn into and out of the cells
question
Explain the movement of K+ during acidosis
answer
The body protects itself from the acidic state by moving H+ ions (from the extracellular fluid) into the cells (intercellular fluid). K+ moves out of the intercellular fluid to make room for the H+ ions and to maintain electrical neutrality The intracellular levels decrease and the extracellular K+ levels increase resulting in hyperkalemia
question
Explain the role of the K+ during alkalosis
answer
In alkalosis the cells release excess intracelluar H+ ions into the blood (the extracellular fluid) in an attempt to increase the acidity of the blood K+ moves into the intercellular fluid to maintain electrical neutrality The intracellular K+ levels increase and the extracellular levels decrease resulting in hypokalemia
question
What are the Priority Nursing Diagnoses with acid base imbalances
answer
Impaired gas exchange Ineffective breathing pattern Ineffective tissue perfusion (pulmonary) Impaired spontaneous ventilation Dysfunctional ventilatory weaning response
question
What is this ABG: pH 7.18 PCO2 38 mm Hg PO2 80 mm Hg HCO3- 15 mEq/L
answer
Uncompensated Metabolic Acidosis TX: Respiratory rate need to increase to blow off C02. May need to give bicarbonate
question
What is this? pH 7.36 PCO2 67 mm Hg PO2 47 mm Hg HCO3 37 mEq/L
answer
Compensated Respiratory Acidosis TX: Patient is hypoxic! ABCs first. May require intubation and a higher respiratory rate
question
What is this? pH 7.60 PCO2 30 mm Hg PO2 70 mm Hg HCO3- 22 mEq/L
answer
Uncompensated Respiratory Alkalosis TX: Slow down respiratory rate. Give 02
question
What is this? pH 7.58 PCO2 35 mm Hg PO2 75 mm Hg HCO3- 50 mEq/L
answer
Uncompensated Metabolic Alkalosis TX: Decrease respiratory rate. Fix underlying issue (May possibly need Fluid and electrolytes)
question
What is this? pH 7.20 PCO2 60 mm Hg PO2 84 mm Hg HCO3- 25 mEq/L
answer
Uncompensated Respiratory Acidosis
question
What is this? pH 7.50 PCO2 44 mm Hg PO2 92 mm Hg HCO3- 32 mEq/L
answer
Uncompensated Metabolic Alkalosis TX: Decrease respiratory rate. Fix underlying issue (May possibly need Fluid and electrolytes)
question
What is this? 33pH 7.33 PCO2 38 mm Hg PO2 82 mm Hg HCO3- 18 mEq/L
answer
Uncompensated Metabolic Acidosis TX: Respiratory rate need to increase to blow off C02. May need to give bicarbonate
question
What is this? pH 7.38 PCO2 52 mm Hg PO2 88 mm Hg HCO3- 29 mEq/L
answer
Compensated Respiratory Acidosis TX: Patient is hypoxic! ABCs first. May require intubation and a higher respiratory rate
question
What is this? pH 7.43 PCO2 28 mm Hg PO2 85 mm Hg HCO3- 20 mEq/L
answer
Compensated Respiratory Alkalosis
question
What is this? pH 7.36 PCO2 32 mm Hg PO2 88 mm Hg HCO3- 20 mEq/L
answer
Compensated Metabolic Acidosis
question
What is this? pH-7.39 PaCO2-48 PaO2-90 HCO3-28
answer
Compensated Respiratory Acidosis
question
What is this? pH-7.48 PaCO2-18 PaO2-94 HCO3-24
answer
Uncompensated Respiratory Alkalosis
question
What is this? pH-7.37 PaCO2-30 PaO2-89 HCO3-21
answer
Compensated Metabolic Acidosis
question
What is this? pH-7.20 PaCO2-75 PaO2-80 HCO3-24
answer
Uncompensated Respiratory Acidosis
question
What is this? pH-7.31 PaCO2-39 PaO2-84 HCO3-19
answer
Uncompensated Metabolic Acidosis
question
What is this? pH-7.42 PaCO2-46 PaO2-88 HCO3-29
answer
Compensated Metabolic Alkalosis
question
What is this? pH-7.44 PaCO2-28 PaO2-92 HCO3-20
answer
Compensated Respiratory Alkalosis
question
What is this? pH-7.30 PaCO2-50 PaO2-89 HCO3-26
answer
Uncompensated Respiratory Acidosis
question
What is this? pH-7.36 PaCO2-30 PaO2-84 HCO3-20
answer
Compensated Metabolic Acidosis
question
What is this? pH-7.50 PaCO2-32 PaO2-85 HCO3-23
answer
Uncompensated Respiratory Alkalosis
question
What is this? pH-7.42 PaCO2-26 PaO2-90 HCO3-21
answer
Compensated Respiratory Alkalosis
question
What is this? pH-7.32 PaCO2-35 PaO2-92 HCO3-20
answer
Uncompensated Metabolic Acidosis
question
What is this? pH 7.43 PaCO2-47 PaO2-82 HCO3-28
answer
Compensated Metabolic Alkalosis
question
Analyze the following ABG: pH: 7.44 PaCO2: 35 mmHg [HCO3-]: 23 mEq/L
answer
Normal All levels are within normal limits. This patient is fine, or at least not suffering from an acid-base imbalance.
question
What is this? pH: 7.27 PaCO2: 19 mmHg HCO3: 8 mEq/L
answer
Uncompensated Metabolic Acidosis Primary problem: Metabolic Acidosis The pH and HCO3- levels are both acidotic, so the primary mechanism is a metabolic acidosis
question
Analyze the following ABG: pH: 7.39 PaCO2: 40 mmHg [HCO3-]: 23 mEq/L
answer
Normal All levels are within normal limits. This patient is fine, or at least not suffering from an acid-base imbalance.
question
Analyze the following ABG: pH: 7.22 PaCO2: 28 mmHg [HCO3-]: 11 mEq/L
answer
Uncompensated Metabolic Acidosis Primary problem: Metabolic Acidosis The pH and HCO3- levels are both acidotic, so the primary mechanism is a metabolic acidosis
question
Analyze the following ABG: pH: 7.30 PaCO2: 16 mmHg HCO3-: 8 mEq/L
answer
Uncompensated Metabolic Acidosis
question
Analyze the following ABG: pH: 7.33 PaCO2: 48 mmHg [HCO3-]: 24 mEq/L
answer
Uncompensated Respiratory Acidosis Primary problem: Respiratory Acidosis The pH and CO2 levels are both acidotic, so the primary mechanism is a respiratory acidosis
question
Analyze the following ABG: pH: 7.23 PaCO2: 36 mmHg [HCO3-]: 15 mEq/L
answer
Uncompensated Metabolic Acidosis Primary problem: Metabolic Acidosis The pH and HCO3- levels are both acidotic, so the primary mechanism is a metabolic acidosis
question
Analyze the following ABG: pH: 7.48 PaCO2: 40 mmHg [HCO3-]: 29 mEq/L
answer
Compensated Metabolic Alkalosis Primary problem: Metabolic Alkalosis The pH and HCO3- levels are both alkalotic, so the primary mechanism is a metabolic alkalosis
question
Analyze the following ABG: pH: 7.50 PaCO2: 17 mmHg [HCO3-]: 13 mEq/L
answer
Uncompensated Respiratory Alkalosis Primary problem: Respiratory Alkalosis The pH and CO2 levels are both alkalotic, so the primary mechanism is a respiratory alkalosis.



