Micro 9: Viral Vectors & Gene Therapy – Flashcards
Unlock all answers in this set
Unlock answersquestion
what is gene therapy?
answer
novel approach to treating disease based on modifying expression of a person's genes toward therapeutic goal
question
first clinical trial for gene therapy? year, strategy, disorder
answer
1990, ex vivo, Adenosine Deaminase Deficiency
question
what was the first in vivo gene therapy treating? what did it use?
answer
cystic fibrosis treated with attenuated adenovirus
question
t/f gene therapy is highly experimental collection of technologies; lots of potential
answer
true
question
what does somatic gene therapy involve?
answer
manipulation of gene expression in cells corrective for patient; will NOT be passed on to further generations
question
what is germline gene therapy?
answer
involves genetic modification of germ cells; these WILL change the next generation
question
which type of therapy somatic/germline, is limited to animal models?
answer
germline
question
steps in ex vivo gene therapy treatment of Familial Hypercholesterolemia
answer
1. remove pt liver, treat cells with retrovirus LDL receptor gene 2. discard liver cells that did not take up corrective genes 3. reimplant corrected genes into patient liver
question
what are the 4 main disease states being targeted for gene therapy intervention?
answer
1 Cancer 2 Monogenic diseases 3 Adenosine deaminase deficiency (SCID) 4 Leber congenital amaurosis
question
what is a vector?
answer
modified, attenuated viruses that are used to deliver payload(gene) into a cell
question
what is a therapeutic strategy?
answer
vector carries a gene that encodes a protein that is defective/not present in host genes
question
what is a cytolytic strategy?
answer
vector is designed to kill or eliminate diseased cell/tissue
question
example of cytolytic therapy; what converts prodrug glancyclovir to toxic drug?
answer
expression of thymidine kinase (TK)
question
What is CAR-T Cell Immunotherapy?
answer
Chimeric Antigen Receptor on a patients Tcell; programming patients own cells to attack cancer
question
Kymriah specifically recognizes what Bcell marker?
answer
CD19
question
with CAR-T Cell Immunotherapy for Acute Lymphoblastic Leukemia: how are Tcells collected? what are they infected with? how are Tcells reintroduced?
answer
-collected by apheresis -infected by viral vector with CAR -the Tcells are reintroduced via Tcell adoptive transfer
question
what group is specifically intended to use Kymriah?
answer
patients with Bcell ALL; intended for patients whose cancer has not responded to treatment
question
where is CD19 found?
answer
surface of differentiated Bcells, NOT hematopoietic stem cells or other essentials
question
how is CD19 utilized in treatment?
answer
CAR binds leukemic Bcells with CD19, then activates Tcells to destroy leukemic Bcells
question
first step in production of CAR?
answer
In recognition domain, variable region gene segments cloned to construct a single-chain variable fragment
question
what is the second step of CAR production?
answer
scFv is fused to extracellular CD8 domain and cytoplasmic CD3
question
what accompanies scFV fusion to CD8?
answer
fuse to transmembrane/ cytoplasmic domain of CD3
question
how do you get CAR into patient's Tcells?
answer
retrovirus, viral vector used to transduce Tcells ex vivo
question
why are retroviruses used to integrate patient's Tcells?
answer
CAR gene will integrate into genome of Tcell
question
what are the steps involved in development of a genetic therapy?
answer
1 identify a gene responsible for dz; clone functional cDNA 2 delivery 3 control gene expression 4 avoid host immune response
question
what is the goal of delivery?
answer
get genetic material to the appropriate cell types: specific, efficient, safe
question
what are most vectors based on?
answer
attenuated or modified versions of viruses
question
what is the challenging part of delivery?
answer
remove disease causing components of virus and insert functional clones to treat
question
what is involved in controlled gene expression?
answer
-making the correct amount of therapy protein, @ right time -maintain longterm expression
question
when avoiding host immune response, what kind of vectors should be used?
answer
vectors that give minimal/no adverse immune response
question
5 types of vectors
answer
adenovirus adeno-associated virus herpes virus liposomes/naked DNA retrovirus
question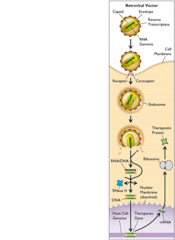
what is a retrovirus?

answer
enveloped virus with RNA genome; upon infection of cell converted to dsDNA by enzyme reverse transcriptase
question
what allows for long-term expression of therapeutic drug during cell division?
answer
viral dsDNA insertion into genome
question
how do they generate a gene therapy vector with retroviruses?
answer
viral genes encoding structural proteins are deleted and replaced with therapeutic gene of interest
question
what is a disadvantage of murine-based retrovirus vectors?
answer
require replicating cells for genome integration
question
what is a Lentivirus? what is the advantage of using it as a vector?
answer
HIV-based gene vector that can integrate into both replicating and non-replicating cells
question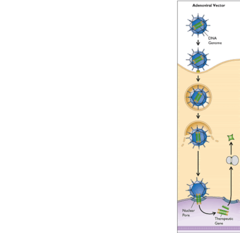
what is an adenovirus?

answer
non-enveloped virus with large dsDNA genome that accommodates large inserts
question
how does an adenovirus enter cell?
answer
binds to specific receptors, delivered to nucleus following uncoating; replicates episomally
question
which proteins are deleted for adenoviruses?
answer
structural proteins and proteins that induce cell division
question
what is a liposome vector?
answer
synthetic lipid that binds to and encapsulates plasmid DNA; fuse with cell membrane and deliver genetic payload
question
why is it that liposomes can deliver to many cell types?
answer
do not bind to specific cell receptors
question
why are structural proteins removed when making a vector?
answer
eliminates the possibility that the vector can become a self-replicating virus
question
first step in making a retroviral vector ends in "packaging cell line"; what is the process?
answer
-genes encoding structural proteins deleted from genome and cloned into plasmid expression vector -plasmid is then transfected into cell; express structural proteins not viral genomes (done in cell culture)
question
what is the second step in making a retroviral gene therapy vector?
answer
sequences from virus that result in packaging of genetic material into virus (aka packaging signals) are removed, put in plasmid -therapeutic gene of interest is cloned between packaging signals
question
what is the third step in retroviral vectors ending in the "producer cell line"?
answer
plasmid with packaging signals introduced into packaging cell line, structural proteins assemble therapeutic gene into virus particles
question
what is the fourth step in making retroviral vectors?
answer
virus released from packaging cell to infect new cells; only delivers therapeutic gene into cells
question
first problem with gene therapy that needs to be solved?
answer
how to avoid an immune response; inflammation or antibodies
question
second problem with gene therapy that needs to be solved?
answer
how to get genes efficiently into non-dividing cells (liver, muscle, neurons)
question
what is the third problem associated with gene therapy that needs to be solved?
answer
how to get genes integrated so that they will not be deleted or over-expressed
question
what is adeno-associated virus (AAV)?
answer
small virus which infects humans and some other primate species
question
how did AAV get its name? why is it advantageous as a gene therapy vector
answer
found in cells simultaneously infected with adenovirus; by itself it is harmless
question
how does AAV differ from adenovirus? (3)
answer
-does not stimulate inflammation in the host -does not elicit antibodies against self -can integrate on chr19, but inefficiently
question
what is Glybera?
answer
AAV1 carrying lipoprotein lipase gene
question
with LPL deficiency patient cannot break down _______; the clinical presentation is (4)
answer
pt cannot break down fats; - abdominal pain -acute and recurrent pancreatitis -eruptive cutaneous xanthoma -hepatosplenomegaly
question
Success with gene therapy for SCID-X1 disease: deficiency? result? what vector was used?
answer
-deficiency of gamma-subunit for IL-2, 4, 7, 9, 15 -causes block T and NK cell differentiation -retrovirus vector encoding gamma-subunit and exvivo CD34+ cells
question
what is the success rate of gene therapy for SCID-X1?
answer
10/11 infants cured
question
Adenosine deaminase deficiency SCID? deficiency? result? vector used?
answer
-purine metabolism defect -impaired lymphocyte development and function -retrovirus vector containing ADA cDNA and exvivo CD34+ cells
question
with correction of ADA-SCID, what did patients undergo before receiving gene therapy?
answer
patients underwent mild nonmyeloablative condtioning with busulfan
question
what is the success rate of gene therapy for ADA-SCID?
answer
both patients treated in this specific study are home and well
question
Leber congenital amaurosis cause? result? vector used?
answer
-bialleic mutations in RPE65 gene -blinding retinal diseases -rAAV2 carrying human RPE65 gene
question
what was the outcome of LCA treatment with rAAV2?
answer
all 12 patients responded well; 6 restored vision, not legally blind
question
who was the first death directly attributed to gene therapy? what did the patient have? what treatment did the patient receive?
answer
Jesse Gelsinger had ornithine transcrabamylase (OTC) deficiency; he was infused with an adenovirus derived vector directly into hepatic artery
question
what was the suggested cause of Jesse Gelsinger's death?
answer
multiorgan failure from anoxia; levels of cytokines suggest systemic inflammatory response syndrome(SIRS)
question
how long after XSCID study did patients begin to develop leukemia?
answer
3 years after treatment
question
strong evidence leads investigators to believe leukemia is due to "___________ _________________"
answer
insertional oncogenesis
question
why is "insertional oncogenesis" considered an unlikely but possible risk for this gene therapy?
answer
vectors cannot reproduce themselves and so cannot repeatedly insert into cell's chromosomes...
question
what is Clustered Regularly Interspaced Short Palindromic Repeats CRISPR/Cas9?
answer
segments of bacterial DNA containing short, repetitive base sequences that form hairpin structures
question
the _____________ sequence precedes the hairpin; it confers sequence _______________
answer
target sequence sequence specificity
question
what makes up the Guide RNA?
answer
target sequence + hairpin structure
question
what is a Cas protein? how does it work with target sequence?
answer
Cas proteins are endonucleases; together with target sequence they recognize and cut exogenous DNA
question
what does the CRSPR/Cas9 system have the capability to do
answer
edit genomic DNA of any species by delivering Cas9 nuclease+ synthetic RNA into cell
question
what are 5 factors influencing development of gene therapy??
answer
1 optimize vector safety 2 overcome tech obstacles 3 analysis of human genome 4 expansion of diagnosis industry 5 public acceptance of gene therapy



