CHEMISTRY EDEXCEL Topic 6: Organic Chemistry – Flashcards
Unlock all answers in this set
Unlock answersquestion
Hydrocarbon
answer
Hydrocarbon is a compound consisting of hydrogen and carbon only
question
Saturated
answer
Contain single carbon-carbon bonds only
question
Unsaturated
answer
Contains a C=C double bond
question
Molecular formula
answer
The formula which shows the actual number of each type of atom
question
Empirical formula
answer
shows the simplest whole number ratio of atoms of each element in the compound
question
General formula
answer
algebraic formula for a homologous series e.g. CnH2n
question
Structural formula
answer
shows the minimal detail that shows the arrangement of atoms in a molecule, eg for butane: CH3CH2CH2CH3 or CH3(CH2)2CH3,
question
Displayed formula
answer
show all the covalent bonds present in a molecule
question
Skeletal formula
answer
shows the simplified organic formula, shown by removing hydrogen atoms from alkyl chains, leaving just a carbon skeleton and associated functional Groups.
question
Homologous series
answer
families of organic compounds with the same functional group and same general formula. •They show a gradual change in physical properties (e.g. boiling point). • Each member differs by CH2 from the last. • same chemical properties.
question
Functional group
answer
is an atom or group of atoms which when present in different molecules causes them to have similar chemical properties
question
Alkane Structure/Prefix
answer
-ane
question
Alkenes Structure/Prefix
answer
-ene
question
Alcohols Structure/Prefix
answer
-ol
question
Halogenoalkanes Structure/Prefix
answer
chloro- bromo- iodo-
question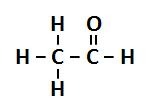
Aldehydes Structure/Prefix
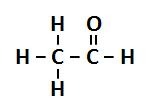
answer
-al
question
Ketones Structure/Prefix
answer
-one
question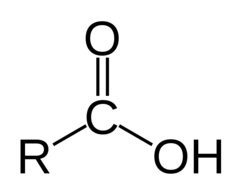
carboxylic acids Structure/Prefix

answer
-oic acid
question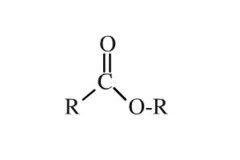
Esters Structure/Prefix

answer
-yl -oate
question
General rules for naming carbon chains
answer
- Count the longest carbon chain and name appropriately - Find any branched chains and count how many carbons they contain - Add the appropriate prefix for each branch chain E.G 3,5-dimethylheptane
question
Basic rules for naming functional groups
answer
- When using a suffix, add in the following way : If the suffix starts with a vowel- remove the -e from the stem alkane name e.g. Propan-1-ol, butan-1-amine, ethanoic acid, ethanoylchloride, butanamide - If the suffix starts with a consonant or there are two or more of a functional group meaning di, or tri needs to be used then do not remove the the -e from the stem alkane name e.g. Propanenitrile, ethane-1,2-diol, propanedioic acid, propane-1,2,3-triol, Pentane-2,4-dione.
question
Isomers
answer
- Structural isomers: same molecular formula different structures (or structural formulae) - Chain isomers: Compounds with the same molecular formula but different structures of the carbon skeleton - Position isomers: Compounds with the same molecular formula but different structures due to different positions of the same functional group on the same carbon skeleton - Functional group isomers: Compounds with the same molecular formula but with atoms arranged to give different functional groups
question
Stereoisomerism
answer
Stereoisomers have the same structural formulae but have a different spatial arrangement of atoms. E-Z stereoisomers arise when: (a) There is restricted rotation around the C=C double bond. (b) There are two different groups/atoms attached both ends of the double bond. E-Z isomers exist due to restricted rotation about the C=C bond Priority Group: The atom with the bigger atomic number is classed as the priority atom
question
Refining crude oil
answer
Fractional Distillation: • Oil is pre-heated • then passed into column. • The fractions condense at different heights • The temperature of column decreases upwards • The separation depends on boiling point. • Boiling point depends on size of molecules. • The larger the molecule the larger the London forces • Similar molecules (size, bp, mass) condense together • Small molecules condense at the top at lower temperatures • and big molecules condense at the bottom at higher temperatures.
question
Cracking
answer
Cracking: conversion of large hydrocarbons to smaller molecules by breakage of C-C bonds
question
Economic reasons for catalytic cracking
answer
• The petroleum fractions with shorter C chains (e.g. petrol and naphtha) are in more demand than larger fractions. • To make use of excess larger hydrocarbons and to supply demand for shorter ones, longer hydrocarbons are cracked. • The products of cracking are more useful and valuable than the starting materials (e.g. ethene used to make poly(ethene) and ethane-1,2-diol, and ethanol) The smaller alkanes are used for motor fuels which burn more efficiently.
question
Reforming
answer
Turns straight chain alkanes into branched and cyclic alkanes and Aromatic hydrocarbons
question
Complete Combustion
answer
In excess oxygen alkanes will burn with complete combustion The products of complete combustion are CO2 and H2O. C8H18(g) + 12.5 O2(g) --> 8CO2(g) + 9 H2O(l)
question
Incomplete Combustion
answer
If there is a limited amount of oxygen then incomplete combustion occurs, producing CO (which is very toxic) and/or C (producing a sooty flame) CH4(g) + 3/2 O2(g) --> CO(g) + 2 H2O(l) CH4(g) + O2(g) --> C(s) + 2 H2O(l)
question
Pollution from Combustion
answer
Sulphur containing impurities are found in petroleum fractions which produce SO2 when they are burned. S+ O2 --> SO2 CH3SH+ 3O2 --> SO2 + CO2 + 2H2O
question
Pollutant and Environmental consequence
answer
- Nitrogen oxides (formed when N2 in the air reacts at the high temperatures and spark in the engine): NO is toxic and can form smog NO2 is toxic and acidic and forms acid rain - Carbon monoxide: toxic - Carbon dioxide: Contributes towards global warming - Unburnt hydrocarbons (not all fuel burns in the engine): Contributes towards formation of smog - Soot/particulates: Global dimming and respiratory problems
question
Catalytic converters
answer
These remove CO, NOx and unburned hydrocarbons (e.g. octane, C8H18) from the exhaust gases, turning them into 'harmless' CO2, N2 and H2O.
question
Biofuels
answer
Most fossil fuels come from crude oil, which is a non- renewable resource. Fossil fuel reserves will eventually run out Alternative fuels have been developed from renewable resources. Alcohols and biodiesel, which can both be made from plants, are two examples of renewable plant- based fuels
question
Advantages of using Biofuels
answer
Reduction of use of fossil fuels which are finite resources biofuels are renewable Use of biodiesel is more carbon-neutral Allows fossil fuels to be used as a feedstock for organic compounds No risk of large scale pollution from exploitation of fossil fuels
question
Disadvantages of Biofuels
answer
Less food crops may be grown Land not used to grow food crops Rain forests have to be cut down to provide land Shortage of fertile soils
question
Mechanisms
answer
To understand how the reaction proceeds we must first understand how bonds are broken in organic mechanisms There are two ways to break a covalent bond: 1. HOMOLYTIC FISSION: each atom gets one electron from the covalent bond 2. HETEROLYTIC FISSION: (one atom gets both electrons) It proceeds via a series of steps: STEP ONE: Initiation STEP TWO: Propagation STEP THREE: Termination
question
Free Radical Substitution Reactions of Alkanes
answer
Overall Reaction CH4 +Cl2 --> CH3Cl+HCl methane --> chloromethane This is the overall reaction, but a more complex mixture of products is actually formed The MECHANISM for this reaction is called a FREE RADICAL SUBSTITUTION
question
STEP ONE: Initiation
answer
Essential condition: UV light The UV light supplies the energy to break the Cl-Cl bond. It is broken in preference to the others as it is the weakest. UV light does not have enough energy to break the C-H bond. The bond has broken in a process called homolytic fission. When a bond breaks by homolytic fission it forms Free Radicals. Free Radicals do not have a charge and are represented by a (black dot). A Free Radical is a reactive species which possess an unpaired electron.
question
STEP TWO: Propagation
answer
CH4 + Cl. --> HCl +.CH3 .CH3 + Cl2 --> CH3Cl + Cl. The chlorine free radicals are very reactive and remove an H from the methane leaving a methyl free radical. The methyl free radical reacts with a Cl2 molecule to produce the main product and another Cl free radical. All propagation steps have a free radical in the reactants and in the products. As the Cl free radical is regenerated, it can react with several more alkane molecules in a CHAIN REACTION.
question
STEP THREE: Termination
answer
.CH3 + Cl. --> CH3Cl .CH3 + .CH3 --> CH3CH3 Cl. + Cl. --> Cl2 Collision of two free radicals does not generate further free radicals: the chain is TERMINATED. Minor step leading to impurities of ethane in product. Write this step using structural formulae and don't use molecular formulae.
question
Alkenes
answer
C=C double covalent bond consists of one sigma (σ) bond and one pi (π) bond. π bonds are exposed and have high electron density. They are therefore vulnerable to attack by species which 'like' electrons: these species are called electrophiles.
question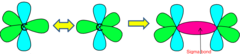
Formation of σ bond

answer
One sp2 orbital from each carbon overlap to form a single C-C bond called a sigma σ bond. Rotation can occur around a sigma bond
question
Formation of π bond
answer
The π bond is formed by sideways overlap of two p orbitals on each carbon atom forming a π-bond above and below the plane of molecule. The π bond is weaker than the σ bond. The pi bond leads to resultant high electron density above and below the line between the two nuclei
question
Reaction of Alkenes with Hydrogen

answer
Change in functional group: alkene --> alkane Reagent: hydrogen Conditions: Nickel Catalyst Type of reaction: Addition/Reduction Electrophilic Addition: Reactions of Alkenes A π bond is weaker than a σ bond so less energy is needed to break π bond The π bonds in alkenes are areas with high electron density. This is more accessible to electrophilic attack by electrophiles. Alkenes undergo addition reactions.
question
Reaction of Alkenes with bromine/chlorine
answer
Change in functional group: alkene --> dihalogenoalkane Reagent: Bromine (dissolved in organic solvent) Conditions: Room temperature (not in UV light) Mechanism: Electrophilic Addition Type of reagent: Electrophile, Br+ Type of Bond Fission: Heterolytic
question
Reaction of Hydrogen Bromide with alkenes
answer
Change in functional group: alkene --> halogenoalkane Reagent: HCl or HBr Conditions: Room temperature Mechanism: Electrophilic Addition Type of reagent: Electrophile, H+ Type of Bond Fission: Heterolytic The order of stability for carbocations is tertiary > secondary > primary In electrophilic addition to alkenes, the major product is formed via the more stable carbocation intermediate.
question
Reaction of Potassium Manganate(VII) with Alkenes
answer
Change in functional group: alkene --> diol Reagent: KMnO4 in an acidified solution Conditions: Room temperature Type of reaction: Oxidation Observation: purple colour of MnO4- ion will decolourise to colourless
question
Reaction of Bromine Water with Alkenes
answer
Reagent: Bromine dissolved in water Conditions: Room temperature Type of reaction: Addition Observation: Orange colour of bromine water will decolourise to colourless
question
hydration of alkenes to form alcohols
answer
Industrially alkenes are converted to alcohols in one step rather than the two in the above sulphuric acid reaction. They are reacted with water in the presence of an acid catalyst. Essential Conditions High temperature 300 to 600°C High pressure 70 atm Catalyst of concentrated H3PO4 This reaction can be called hydration: a reaction where water is added to a molecule. The high pressures needed mean this cannot be done in the laboratory. It is preferred industrially, however, as there are no waste products and so has a high atom economy. It would also mean separation of products is easier (and cheaper) to carry out. See equilibrium chapter for more on the industrial conditions for this reaction.
question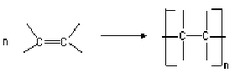
Addition Polymers

answer
Addition polymers are formed from alkenes This is called addition polymerisation. Poly(ethene): is used to make plastics bags, buckets, bottles. It is a flexible, easily moulded, waterproof, chemical proof, and low density plastic. Poly(propene) is a stiffer polymer, used in utensils and containers and fibres in rope and carpets.
question
Incineration
answer
Rubbish is burnt and energy produced is used to generate electricity. Some toxins can be released on incineration. (e.g. Combustion of halogenated plastics (ie PVC) can lead to the formation of toxic, acidic waste products such as HCl.) Modern incinerators can burn more efficiently and most toxins and pollutants can be removed. Greenhouse gases will still be emitted though. Volume of rubbish is greatly reduced.
question
Recycling
answer
Saves raw materials- nearly all polymers are formed from compounds sourced/produced from crude oil. Saves precious resources. Polymers need collecting/ sorting- expensive process in terms of energy and manpower. Polymers can only be recycled into the same type - so careful separation needs to be done. Thermoplastic polymers can be melted down and reshaped.
question
feedstock for cracking
answer
Polymers can be cracked into small molecules which can be used to make other chemicals and new polymers- Saves raw materials-
question
Naming Halogenoalkanes
answer
Based on original alkane, with a prefix indicating halogen atom: Fluoro for F; Chloro for Cl; Bromo for Br; Iodo for I. Halogenoalkanes can be classified as primary, secondary or tertiary depending on the number of carbon atoms attached to the C-X functional group.
question
Primary halogenoalkane
answer
One carbon attached to the carbon atom adjoining the halogen
question
Secondary halogenoalkane
answer
Two carbons attached to the carbon atom adjoining the halogen
question
Tertiary halogenoalkane
answer
Three carbons attached to the carbon atom adjoining the halogen
question
Reactions of Halogenoalkanes
answer
Halogenoalkanes undergo either substitution or elimination reactions.
question
Nucleophilic substitution reactions
answer
Substitution: swapping a halogen atom for another atom or groups of atoms. Nucleophile: electron pair donator e.g. :OH-, :NH3, CN-. :Nu represents any nucleophile - they always have a lone pair and act as electron pair donators. The rate of these substitution reactions depends on the strength of the C-X bond. The weaker the bond, the easier it is to break and the faster the reaction. The iodoalkanes are the fastest to substitute and the fluoroalkanes are the slowest. The strength of the C-F bond is such that fluoroalkanes are very unreactive
question
Comparing the rate of hydrolysis reactions
answer
Hydrolysis is defined as the splitting of a molecule ( in this case a halogenoalkane) by a reaction with water. Water is a poor nucleophile but it can react slowly with halogenoalkanes in a substitution reaction. Aqueous silver nitrate is added to a halogenoalkane and the halide leaving group combines with a silver ion to form a SILVER HALIDE PRECIPITATE. The precipitate only forms when the halide ion has left the halogenoalkane and so the rate of formation of the precipitate can be used to compare the reactivity of the different halogenoalkanes. The quicker the precipitate is formed, the faster the substitution reaction and the more reactive the haloalkane The rate of these substitution reactions depends on the strength of the C-X bond . The weaker the bond, the easier it is to break and the faster the reaction. AgI (s) - yellow precipitate (forms fastest) AgBr(s) - cream precipitate AgCl(s) - white precipitate
question
Nucleophilic substitution with aqueous hydroxide ions
answer
Change in functional group: halogenoalkane --> alcohol Reagent: potassium (or sodium) hydroxide Conditions: In aqueous solution; Heat under reflux Mechanism: Nucleophilic Substitution Role of reagent: Nucleophile, OH- The aqueous conditions needed is an important point. If the solvent is changed to ethanol an elimination reaction occurs
question
SN1 nucleophilic substitution mechanism for tertiary halogenoalkanes
answer
Tertiary halogenoalkanes undergo this mechanism as the tertiary carbocation is made stabilised by the electron releasing methyl groups around it. (see alkenes topic for another example of this). Also the bulky methyl groups prevent the hydroxide ion from attacking the halogenoalkane in the same way as the mechanism above. Primary halogenoalkanes don't do the SN1 mechanism because they would only form an unstable primary carbocation.
question
Nucleophilic substitution with ammonia
answer
Change in functional group: halogenoalkane --> amine Reagent: NH3 dissolved in ethanol Conditions: Heating under pressure in a sealed tube Mechanism: Nucleophilic Substitution Type of reagent: Nucleophile, :NH3
question
Elimination with alcoholic hydroxide ions
answer
Change in functional group: halogenoalkane --> alkene Reagents: Potassium (or sodium) hydroxide Conditions: In ethanol ; Heat Mechanism: Elimination Role of reagent: Base, OH- Note the importance of the solvent to the type of reaction here. Aqueous: substitution Alcoholic: elimination The structure of the halogenoalkane also has an effect on the degree to which substitution or elimination occurs in this reaction. Primary tends towards substitution Tertiary tends towards elimination
question
Uses of halogenoalkanes
answer
Haloalkanes have been used as refrigerants, fire retardants, pesticides and aerosol propellants chloroalkanes and chlorofluoroalkanes can be used as solvents. Many of these uses have now been stopped due to the toxicity of halogenoalkanes and also their detrimental effect on the ozone layer.
question
Naming Alcohols
answer
These have the ending -ol and if necessary the position number for the OH group is added between the name stem and the -ol If the compound has an -OH group in addition to other functional groups that need a suffix ending then the OH can be named with the prefix hydroxy-): If there are two or more -OH groups then di, tri are used. Add the 'e' on to the stem name though
question
Bond angles in Alcohols
answer
The HCH bond has 4 bonded pairs and 0 lone pairs, so the bond angle is 109.5 (tetrahedral). 2. The COH bond has 2 bonded pairs and 2 lone pairs (on the oxygen atom), so the bond angle is 104.5 (V-shaped or bent).
question
Different types of alcohols
answer
- Primary alcohols are alcohols where 1 carbon is attached to the carbon adjoining the oxygen - Secondary alcohols are alcohols where 2 carbon are attached to the carbon adjoining the oxygen - Tertiary alcohols are alcohols where 3 carbon are attached to the carbon adjoining the oxygen
question
1. Combustion of Alcohols
answer
Alcohols combust with a clean flame
question
2. Reaction of Alcohols with Sodium
answer
Sodium reacts with alcohols 2CH CH OH + 2Na --> 2CH3CH2O-Na+ + H2 This reaction can be used as a test for alcohols Observations: • effervescence, • the mixture gets hot, • sodium dissolves, • a white solid is produced.
question
3. Substitution reactions of Alcohols to form Halogenoalkanes
answer
This reaction with PCl5 (phosphorous(v)chloride) can be used as a test for alcohols. You would observe misty fumes of HCl produced. For Br use KBr, 50% concentrated H2SO4 to produce HBr. The reaction of KI and conc H2SO4 cant be used to produce HI because the sulphuric acid with oxidise the hydrogen halides to other products.
question
4. Oxidation reactions of the alcohols
answer
Potassium dichromate K2Cr2O7 is an oxidising agent that causes alcohols to oxidise. The exact reaction, however, depends on the type of alcohol, i.e. whether it is primary, secondary, or tertiary, and on the conditions. -- Partial Oxidation of Primary Alcohols: Reaction: primary alcohol --> aldehyde Reagent: potassium dichromate (VI) solution and dilute sulphuric acid. Conditions: (use a limited amount of dichromate) warm gently and distil out the aldehyde as it forms: -- Full Oxidation of Primary Alcohols: Reaction: primary alcohol --> carboxylic acid Reagent: potassium dichromate(VI) solution and dilute sulphuric acid Conditions: use an excess of dichromate, and heat under reflux: (distill off product after the reaction has finished) -- Oxidation of Secondary Alcohols: Reaction: secondary alcohol --> ketone Reagent: potassium dichromate(VI) solution and dilute sulphuric acid. Conditions: heat under reflux
question
Distinguishing between Aldehydes and Ketones
answer
The fact that aldehydes can be further oxidised to carboxylic acids whereas ketones cannot be further oxidised is the chemical basis for tests that are commonly used to distinguish between aldehydes and ketones. Fehling's (Benedict's) solution Reagent: Fehling's Solution containing blue Cu 2+ ions. Conditions: heat gently Reaction: aldehydes only are oxidised by Fehling's solution into a carboxylic acid and the copper ions are reduced to copper(I) oxide Observation: Aldehydes :Blue Cu 2+ ions in solution change to a red precipitate of Cu2O. Ketones do not react
question
Reaction of Alcohols with Dehydrating Agents
answer
Reaction: Alcohol --> Alkene Reagents: Concentrated Phosphoric acid Conditions: warm (under reflux) Role of reagent: dehydrating agent/catalyst Type of reaction: acid catalysed elimination Producing alkenes from alcohols provides a possible route to polymers without using monomers derived from oil
question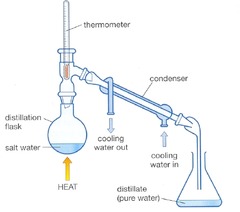
Distillation

answer
In general used as separation technique to separate an organic product from its reacting mixture. Need to collect the distillate of the approximate boiling point range of the desired liquid. Classic AS reaction using distillation Reaction: primary alcohol --> aldehyde Reagent: potassium dichromate (VI) solution and dilute sulphuric acid. Conditions: use a limited amount of dichromate and warm gently and distil out the aldehyde as it forms [This prevents further oxidation to the carboxylic acid] CH3CH2CH2OH + [O] --> CH3CH2CHO + H2O Observation: Orange dichromate solution changes to green colour of Cr3+ ions
question
Reflux
answer
Reflux is used when heating organic reaction mixtures for long periods. The condenser prevents organic vapours from escaping by condensing them back to liquids. Never seal the end of the condenser as the build up of gas pressure could cause the apparatus to explode. This is true of any apparatus where volatile liquids are heated. Classic AS reaction using reflux Reaction: primary alcohol --> carboxylic acid Reagent: potassium dichromate(VI) solution and dilute sulphuric acid Conditions: use an excess of dichromate, and heat under reflux: (distill off product after the reaction has finished using distillation set up) CH3CH2CH2OH + 2[O] --> CH3CH2CO2H + H2O Observation: Orange dichromate solution changes to green colour of Cr3+ ions
question
Purifying an organic liquid
answer
• Put the distillate of impure product into a separating funnel • wash product by adding either - sodium hydrogencarbonate solution , shaking and releasing the pressure from CO2 produced. Sodium hydrogencarbonate will neutralise any remaining reactant acid. - Saturated sodium chloride solution. •Allow the layers to separate in the funnel, and then run and discard the aqueous layer. •Run the organic layer into a clean, dry conical flask and add three spatula loads of drying agent (anhydrous sodium sulphate) to dry the organic liquid. • Carefully decant the liquid into the distillation flask •Distill to collect pure product
question
Solvent extraction
answer
Mix organic solvent and oil-water mixture in a separating funnel then separate the oil layer. Distil to separate oil from organic solvent Add anhydrous CaCl2 to clove oil to dry oil Decant to remove CaCl2
question
Measuring boiling point
answer
Purity of liquid can be determined by measuring a boiling point. This can be done in a distillation set up or by simply boiling a tube of the sample in an heating oil bath. To get a correct measure of boiling point the thermometer should be above the level of the surface of the boiling liquid and be measuring the temperature of the saturated vapour. Pressure should be noted as changing pressure can change the boiling point of a liquid. Measuring boiling point is not the most accurate method of identifying a substance as several substances may have the same boiling point.



