Kaplan Organic Chemistry Chapter 3 : Bonding – Flashcards
Unlock all answers in this set
Unlock answersquestion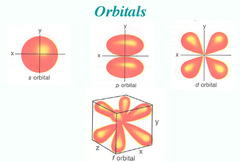
Atomic Orbitals

answer
the regions around the nucleus within which the electrons have the highest probability of being found
question
Principal Quantum Number - n
answer
Number that corresponds to the energy level of a given electron in an atom, is essentially a measure of size The smaller the number the closer the shell is to the nucleus and thus has lower energy, Values are up to 7 on the MCAT
question
Azimuthal Quantum Number - l
answer
Number that corresponds to the number of subshells in an each electron shell Ranges from 0 --> n-1 Energy increases as the azimuthal quantum number increases
question
Magnetic Quantum Number - ml
answer
Number that corresponds to the orbitals within each subshell Ranges from -l to +l There are several types of orbitals each with a specific shape
question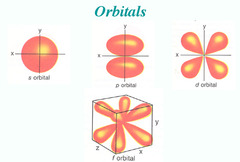
s-orbital

answer
An atomic orbital that is spherical and symmetrical, centered on the nucleus
question
p-orbital
answer
An atomic orbital that is composed of two lobes located symmetrically about the nucleus and contains a node
question
Node
answer
Area where the probability of finding an electron is zero
question
d-orbital
answer
An atomic orbital that is composed of four symmetrical lobes and contains two nodes
question
Spin quantum number - ms
answer
Each orbital can hold two electrons, which is distinguished by this.
question
Molecular Orbitals
answer
Occurs when 2 atomic orbitals combine; apply to the entire molecule Obtained mathematically by adding or subtracting the wave functions of atomic orbitals
question
Bonding Orbital
answer
Occurs when the signs of the wave functions are the same, are lower energy, more stable molecular orbital that can be occupied by two electrons of a covalent bond
question
Antibonding Orbital
answer
Occurs when the signs of the wave functions are different, are higher energy, less stable A molecular orbital formed by the overlap of two or more atomic orbitals; energy is greater than the energy of the combining atomic orbitals
question
Sigma Bond
answer
a bond formed when two atomic orbitals form head-to-head or tail-to-tail overlap, these atomic orbitals combine to form a molecular orbital that is symmetrical around the axis connecting the two atomic nuclei All single bonds are sigma bonds
question
Pi Bond
answer
Bond formed when two p-orbitals line up in a parallel fashion, electron clouds overlap forming the pi-bond Cannot exist independently of a sigma bond Prevents free rotation around the bond axis A Pi-bond on top of an existing sigma bond forms a double bond 2 Pi-bonds with a sigma bond form a triple bond
question
Double Bond
answer
Formed by a single pi bond along with a sigma bond Involves the sharing of 2 pairs of electrons between two atoms Involved in resonance resulting in more rigid structures
question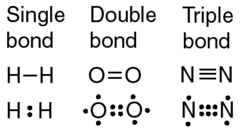
Triple Bond
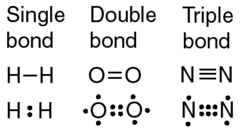
answer
Formed by 2 pi bonds along with a sigma bond Involves the sharing of 3 pairs of electrons between two atoms Shortest in length, requires the most energy to break Involved in resonance resulting in more rigid structures
question
Hybridization
answer
Several atomic orbitals mix to form the same total number of equivalent hybrid orbitals
question
Hybrid Orbitals
answer
An orbital that results from the mixing of different kinds of atomic orbitals
question
S character
answer
Fraction of a hybrid orbital that corresponds to an s orbital; about one half for sp, one third for sp2 and one forth for sp3
question
sp3
answer
Hybrid atomic orbital formed by the combination of one s atomic orbital and three p atomic orbitals. Creating 4 sp3 hybridized orbitals This hybridization is commonly seen in alkanes, or carbons with 4 substiuent groups. 25% s character, 75% p character Tetrahedral Bond angles of 109.5 degrees Example : Methane
question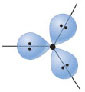
sp2

answer
Hybrid atomic orbital formed by the combination of one s atomic orbital and 2 p atomic orbitals. Creating 3 sp2 hybridized orbitals This hybridization is commonly seen in alkenes, or atoms possessing a double bond. 33% s character, 67% p character Trigonal Planar Bond angles of 120 degrees Example : Alkenes
question
sp hybridization
answer
Hybrid atomic orbital formed by the combination of one s atomic orbital and 2 p atomic orbitals. Creating 2 sp hybridized orbitals This hybridization is commonly seen in alkynes, or atoms with triple bonds, or 2 double bonds. 50% s character, 50%p character Linear Bond angles of 180 degrees Example : Alkynes, CO2
question
Resonance
answer
Delocalization of electrons causing partial double bond character, often seen in aromatic rings, or conjugated hydrocarbons
question
Conjugation
answer
Alternating single and multiple bonds, delocalization of these electrons will cause shared bond characters. The electron density is shared amongst the structure If forms of resonant structures have differing stability, electron density will favor the most stable form Allows for more rigid structures



