Things to Remember: Organic Chemistry – Flashcards
Unlock all answers in this set
Unlock answersquestion
Isopropyl alcohol
answer
also 2-propanol
question
Ethanol
answer
also known as ethyl alcohol
question
Structural isomers
answer
-only thing in common is molecular weight -different atomic connectivity -have different physical and chemical properties -no similarity in structures
question
Stereoisomers
answer
-same atomic connectivity (same structural backbone) -differ in how these atoms are arranged in space (wedged vs dashed) -Conformational (rotation around single bonds) vs Configurational (need to break bonds for interconversion)
question
Conformational isomers
answer
-the most similar out of all the isomers -rotating around single bond, can cause strain some times -use newman projections -anti-Staggered (lowest energy conformation), gauche, totally eclipsed (highest energy conformation, higher the energy the less stable) Cyclic conformations: -axial H's: perpendicular to plane of the ring(sticking up and down) -equatorial: parallel to the plane of the ring(sticking out) -during a chair flip, axial components become equatorial components and vise versa (however if it was up (wedge) to begin with, it stays up, and down (dash) to begin with stays down) -larger groups need to be in equitorial positions
question
Configurational isomers
answer
-can only change from one form to another by breaking bonds -enantiomers and diastereomers (both are optical isomers) -chirality: mirror images cannot be superimposed (carbon with 4 different groups) Enantiomers: non superimposable mirror images of each other, IDENTICAL physical and chemical properties (but they rotate plane-polarized light in opposite directions and react differently in chiral environments) (R and S are opposite between enantiomers) Diastereomers: chiral, same connectivity, NOT mirror images (differ at some chiral centers), non superimposable, need multiple chiral centers obvs DIFFERENT chemical and physical properties, R and S are same between diastereomers) -->meso compounds have chiral centers but also have LINE of SYMMETRY (not optically active) Use R and S to determine absolute configurations E opposite side Z zame side achiral molecules can be superimposed
question
Molecular orbitals
answer
can be bonding or antibonding depending on the sign of the wave functions. Head to head or tail to tail overlap of atomic orbitals results in a sigma bond (single bond) one pi bond and one sigma bond is a double bond one sigma bond and two pi bonds are a triple bond individually sigma is strong than pi. however double and triple bonds are stronger than single bonds higher the s character, stronger sp3: 109.5 degrees sp2: 120 degrees sp: 180 degrees
question
Lewis Definitions
answer
Lewis acid: electron pair acceptor (electrophiles) Lewis base: electron pair donor (nucleophiles) Coordinate covalent bond: both electrons in the bond come from the same starting atom
question
Bronsted Lowry definitions
answer
acid is a proton donor, base is a proton acceptor
question
Acid strength
answer
as bond strength decreases, acidity increases, more electronegative higher acidity
question
Nucleophile
answer
-essentially a lewis base -Nucleophilicity increases with increasing electron density (more negative charge) - The less electronegative the better (more likely to share electron density) -Bulkier the molecule, the less nucleophilic -Protic solvents are bad (can protonate the nucleophile) --> In polar protic solvents, nucleophilicity increases down the periodic table --> In polar aprotic solvents, nucleophilicity increases up the periodic table nucleophile strength is a kinetic property (based on relative rates of reaction), basicity is a thermodynamic property (requires equilibrium) you need polar solvents regardless so that nucleophile can dissolve
question
Electrophiles
answer
-essentially a lewis acid -positively charged (like a Carbonyl carbon) electrophilicity strength is a kinetic property (based on relative rates of reaction), basicity is a thermodynamic property (requires equilibrium)
question
Leaving groups
answer
conjugate bases of strong acids make good leaving groups
question
SN1 reaction
answer
1. leaving group leaves --> carbocation 2. nucleophile attacks --> product more substituted the carbocation the more stable (act as electron donors) can form racemic mixtures (carbocation is planar)
question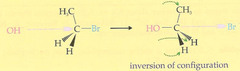
SN2 reactions

answer
1. nucleophile attacks as the leaving group leaves (one step) -concerted reaction -backside attack -less substituted the better (for the nucleophile to attack) - changes absolute configuration
question
Oxidation
answer
Primary/secondary alcohol to aldehyde/ketone (use PCC) Aldehyde/alcohol/alkane --> caboxylic acid (use KMNO4) alkene --> aldehyde/ketone (use O3 and Ch3SCh3) alkene/alkyne --> carb acid/ketone (use O3 and H2O2) alkene --> epoxide (mCPBA)
question
Reduction
answer
aldehyde/ketone --> primary/secondary alcohol (use LAH) amide --> primary amine (use LAH and H+/H2O) carboxylic acid --> primary alcohol (use LAH and H2O) ester --> primary alcohol (use LAH and H2O)
question
Alcohols
answer
-end in -ol or can have "alcohol" is in the name - Hydroxyl hydrogens in phenols are particularly acidic due to resonance within the phenol ring - two groups on adjacent carbons (ortho), separated by a carbon (meta), opposite side of ring (para) - can do intermolecular hydrogen bonding --> higher melting points and boiling points -Phenols are very acidic -Acidity decreases as more alkyl groups are attached because they are more electron-donating (stabilize positive charges) -Resonance/electron withdrawing groups stabilize alkoxide anions, making alcohols more acidic
question
Alcohol reactions
answer
Oxidation Reactions: - Primary Alcohol --> (PCC) --> Aldehyde -Secondary Alcohol --> (Na2Cr2O7/H2SO4) --> Ketone -Primary Alcohol --> (CrO3/H2SO4) --> carboxylic acid Tosylates: make hydroxyl groups of alcohols into better leaving groups for nucleophilic substitution reactions
question
Phenol Reactions
answer
Phenol --> (Na2Cr2O7/H2SO4) --> Ketone
question
Quinones
answer
watch video about it
question
Aldehydes and Ketones
answer
-Both contain a carbonyl group -Aldehydes are always terminal group - Strong smelling -Aldehydes in -al, ketone end in -one -Dipole moments are strong due to the carbonyl groups, but not as significant of the hydrogen bonding in alcohols - The carbonyl carbon is the most common electrophile
question
Nucleophilic Additions of Aldehydes and Ketones
answer
The picture is a hydration reaction Usually the Nu attacks the Carbonyl carbon, the O of the carbonyl grabs an H
question
Oxidation/Reduction of Aldehydes
answer
Use KMnO4, CrO3/ Ag2O,HxO2 to turn aldehydes into carboxylic acids Use LAH for reduction of ketones
question
alpha-Hydrogen of carbonyl containing compounds
answer
-The acidity of the alpha-H allows many aldehydes/ketones to act as both electrophiles and nucleophiles -The alpha-C is adjacent to the carbonyl carbon, the hydrogens connected to it are called alpha-Hydrogens -Because of the Carbonyl Oxygen and its electron withdrawing effects, it weakens the alphaC-H bond, making the alpha-Carbon easy to deprotonate (help stabilize the carboanion intermediate) -The alpha Hydrogens of ketones tend to be less acidic than those of aldehydes due to electron donating properties of the alkyl group of the ketone, the alkyl groups of the on the ketones make it less likely to interact with nucleophiles and destabilizes the carboanion (increasing steric hindrance)
question
Enolate Chemistry
answer
Because of the acidity of the alpha-Hydrogen, aldehydes and ketones exit in solution as a mixture of 2 isomers: keto and enol form -Enol: presence of a C=C double bond and an alcohol -Keto: the presence of a C=O -Both are tautomers and equilibrium lies on the Keto side When deprotonating the alpha-C using a strong base , and forming the enolate carboanion, it can attack electrophiles in the reactions below When a ketone has two different alkyl groups, 2 forms of an enolate can form (a C=C forming between either the less or more substituted carbon). The Kinetically favored product forms faster but is less stable (double bond to the less substituted carbon). Kinetic product is favored at rapid, irreversible, low temp, strong/sterically hindered base reaction. The Thermodynamically favored product forms slower but is more stable (double bond to the more substituted carbon). Thermodynamic product is favored at slow, reversible, high temp, weak/smaller base reactions.
question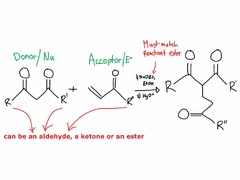
Michael Addition

answer
Carboanion attacks an alpha,beta unsaturated carbonyl compound (double bond).
question
Enamines
answer
Enamines are tautomers of imines (C=N). Imines are favored thermodynamically GO OVER
question
Aldol Condensation
answer
Aldehyde/ketone acts both as electrophile (keto form) and a nucleophile (enol/enolate form) forming a C=C double bond Step 1: forming the aldol (a molecule with both an aldehyde and alcohol) Step 2: dehydration of the aldol, the -OH is removed as water forming an alpha-beta double bond Retro-Aldol Reaction: forming the aldol, but then using a strong base to break the bonds between alpha and beta carbons of a carbonyl (makes to to carbonyl compounds)
question
Carboxylic Acids
answer
- Can act as acids, nucleophiles, and electrophiles, can deprotonate that -OH, have pK around 3-6, excellent at H-bonding and have high boiling points -Always C-1 in a chain, just add "-oic acid" to the end of the name -Good acids since the conjugate base is really stable (resonance)
question
Synthesis of Carboxylic Acids
answer
-Oxidation of aldehydes/primary alcohols using KMnO4
question
Nucleophilic Acyl Substitution
answer
Step 1: Nucleophilic addition Step 2: Elimination of the leaving group (the -OH) and reformation of the carbonyl Pretty much how you form acid derivatives: amines attack to form amides and lactams Esterification: 1. C=O gets protonated, primary alcohol attacks, the -OH of the carboxylic acid takes the H of the alcohol and is the leaving group (water) Anhydrides: Condensation of two carboxylic acids, the -OH of one attacks the Carbonyl carbon of another, add a H+ to create a water on the -OH of the attacked c-acid , water leaves a leaving group
question
Reduction of Carboxylic Acids
answer
Using Hydride ion or LAH, you can reduce a carboxylic acid into a primary alcohol
question
Decarboxylation
answer
Complete loss of carboxyl group as carbon dioxide WATCH VIDEO OVER
question
Saponification
answer
long chain carboxylic acids react with sodium or potassium hydroxide, salt is formed Saponification occurs by mixing fatty acids with (sodium or potassium hydroxide) resulting in the formation of a salt that we call soap
question
Carboxylic Acid Reactivity Principles
answer
More Reactive to Less Reactive: due to amount of electron withdrawing groups Anhydrides ; Esters ; Carboxylic acids ; Amides Steric Hindrance can be used to control where a reaction occurs in a molecule Electronic effects:
question
Strecker Synthesis:
answer
watch video
question
Gabriel Synthesis:
answer
watch video
question
NMR Spectroscopy
answer
watch video



