Quantitative – Flashcards
Unlock all answers in this set
Unlock answersquestion
How many particles in a mole?

answer
6.02x10(23)
question
What are moles a quantity of?

answer
Amount of substance
question
Number of particles =

answer
number of moles x 6.02x10(23)
question
What is molar mass?

answer
Molar mass is the gram equivelent of the relitive atomic mass on the periodic table, the mass of one mole of a substance
question
Mass =

answer
number of moles x molar mass
question
concentration =

answer
number of moles / volume of solution c=n/v
question
one litre =

answer
1dm3 = 1000ml = 1000cm3
question
0 Kelvin =

answer
-273 degrees C
question
1atm

answer
101.325 kPa
question
Pressure =

answer
1 / volume
question
P1V1 =

answer
P2V2
question
Volume =

answer
Temperature
question
V / T =

answer
constant
question
V1/T1 =

answer
V2/T2
question
P1V1/T1 =

answer
P2V2/T2
question
Avogadros Law

answer
Equal volumes of gas contain equal moles of gas
question
1.0 moles of gas occupies

answer
22.4dm3 @ standard conitions
question
PV =

answer
nRT
question
R =

answer
8.314
question
percent yield =
answer
experimental yield / Theoretical Yield x 100%
question
1.7 g of NaNO3 (Mr = 85) is dissolved in water to prepare 0.20 dm3 of solution. What is the concentration of the resulting solution in mol dm-3?

answer
0.1
question
The relative molecular mass of a gas is 56 and its empirical formula is CH2. What is the molecular formula of the gas?

answer
C4H8
question
What mass, in g, of hydrogen is formed when 3 mol of aluminium react with excess hydrochloric acid according to the following equation? 2Al(s) + 6HCl(aq) → 2AlCl3(aq) + 3H2(g)

answer
9.0
question
What is the total number of hydrogen atoms in 1.0 mol of benzamide, C6H5CONH2?

answer
4.2 × 1024
question
A fixed mass of gas has a certain volume at a temperature of 50 °C. What temperature is required to double its volume while keeping the pressure constant?

answer
646 K
question
Chloroethene, C2H3Cl, reacts with oxygen according to the equation below. 2C2H3Cl(g) + 5O2(g) → 4CO2(g) + 2H2O(g) + 2HCl(g) What is the amount, in mol, of H2O produced when 10.0 mol of C2H3Cl and 10.0 mol of O2 are mixed together, and the above reaction goes to completion?

answer
4.00
question
Equal masses of the metals Na, Mg, Ca and Ag are added to separate samples of excess HCl(aq). Which metal produces the greatest total volume of H2(g)?

answer
Mg
question
5 dm3 of carbon monoxide, CO(g), and 2 dm3 of oxygen, O2(g), at the same temperature and pressure are mixed together. Assuming complete reaction according to the equation given, what is the maximum volume of carbon dioxide, CO2(g), in dm3, that can be formed? 2CO(g) + O2(g) → 2CO2

answer
4
question
Which of the following has the greatest mass? A. 6 x 1025 atoms of helium gas B. 10 moles of oxygen molecules C. 1.2 x 1024 atoms of copper D. 1 mole of gold atoms

answer
A. 6 x 1025 atoms of helium gas
question 2BaO + O2 If 0.500 mol of BaO2 is decomposed, the number of moles of O2 formed is" alt="Barium peroxide, BaO2, decomposes when heated to give BaO and O2. 2BaO2 --> 2BaO + O2 If 0.500 mol of BaO2 is decomposed, the number of moles of O2 formed is">
2BaO + O2 If 0.500 mol of BaO2 is decomposed, the number of moles of O2 formed is" alt="Barium peroxide, BaO2, decomposes when heated to give BaO and O2. 2BaO2 --> 2BaO + O2 If 0.500 mol of BaO2 is decomposed, the number of moles of O2 formed is">
Barium peroxide, BaO2, decomposes when heated to give BaO and O2. 2BaO2 --> 2BaO + O2 If 0.500 mol of BaO2 is decomposed, the number of moles of O2 formed is
 2BaO + O2 If 0.500 mol of BaO2 is decomposed, the number of moles of O2 formed is" alt="Barium peroxide, BaO2, decomposes when heated to give BaO and O2. 2BaO2 --> 2BaO + O2 If 0.500 mol of BaO2 is decomposed, the number of moles of O2 formed is">
2BaO + O2 If 0.500 mol of BaO2 is decomposed, the number of moles of O2 formed is" alt="Barium peroxide, BaO2, decomposes when heated to give BaO and O2. 2BaO2 --> 2BaO + O2 If 0.500 mol of BaO2 is decomposed, the number of moles of O2 formed is">answer
0.250
question
Which of the following is an empirical formula? A. Al2Cl6 B. H2C2O4 C. C2H6 D. CH4O

answer
D. CH4O
question KNO3(aq) + AgCl(s)" alt="How many grams of AgCl would be precipitated if an excess of AgNO3 solution were added to 55.0 cm3 of 0.200 M KCl solution? AgNO3(aq) + KCl(aq) --> KNO3(aq) + AgCl(s)">
KNO3(aq) + AgCl(s)" alt="How many grams of AgCl would be precipitated if an excess of AgNO3 solution were added to 55.0 cm3 of 0.200 M KCl solution? AgNO3(aq) + KCl(aq) --> KNO3(aq) + AgCl(s)">
How many grams of AgCl would be precipitated if an excess of AgNO3 solution were added to 55.0 cm3 of 0.200 M KCl solution? AgNO3(aq) + KCl(aq) --> KNO3(aq) + AgCl(s)
 KNO3(aq) + AgCl(s)" alt="How many grams of AgCl would be precipitated if an excess of AgNO3 solution were added to 55.0 cm3 of 0.200 M KCl solution? AgNO3(aq) + KCl(aq) --> KNO3(aq) + AgCl(s)">
KNO3(aq) + AgCl(s)" alt="How many grams of AgCl would be precipitated if an excess of AgNO3 solution were added to 55.0 cm3 of 0.200 M KCl solution? AgNO3(aq) + KCl(aq) --> KNO3(aq) + AgCl(s)">answer
0.2 x (55/1000) = 0.011 mol of KCl Which also = 0.011 mol of AgNO3 Mass = mol x Mr = 0.011 x 143 = 1.573g
question (NH4)2SO4 (aq)" alt="A Household cleaners contains ammonia. 25.37g of this cleaner is dissolved in water and made up to 250 cm3. A 25.0 cm3 portion of this solution requires 37.3 cm3 of 0.360 mol/dm3 sulphuric acid for neutralisation. What is the percentage by mass of ammonia in the cleaner ? 2NH3(aq) + H2SO4(aq) --> (NH4)2SO4 (aq)">
(NH4)2SO4 (aq)" alt="A Household cleaners contains ammonia. 25.37g of this cleaner is dissolved in water and made up to 250 cm3. A 25.0 cm3 portion of this solution requires 37.3 cm3 of 0.360 mol/dm3 sulphuric acid for neutralisation. What is the percentage by mass of ammonia in the cleaner ? 2NH3(aq) + H2SO4(aq) --> (NH4)2SO4 (aq)">
A Household cleaners contains ammonia. 25.37g of this cleaner is dissolved in water and made up to 250 cm3. A 25.0 cm3 portion of this solution requires 37.3 cm3 of 0.360 mol/dm3 sulphuric acid for neutralisation. What is the percentage by mass of ammonia in the cleaner ? 2NH3(aq) + H2SO4(aq) --> (NH4)2SO4 (aq)
 (NH4)2SO4 (aq)" alt="A Household cleaners contains ammonia. 25.37g of this cleaner is dissolved in water and made up to 250 cm3. A 25.0 cm3 portion of this solution requires 37.3 cm3 of 0.360 mol/dm3 sulphuric acid for neutralisation. What is the percentage by mass of ammonia in the cleaner ? 2NH3(aq) + H2SO4(aq) --> (NH4)2SO4 (aq)">
(NH4)2SO4 (aq)" alt="A Household cleaners contains ammonia. 25.37g of this cleaner is dissolved in water and made up to 250 cm3. A 25.0 cm3 portion of this solution requires 37.3 cm3 of 0.360 mol/dm3 sulphuric acid for neutralisation. What is the percentage by mass of ammonia in the cleaner ? 2NH3(aq) + H2SO4(aq) --> (NH4)2SO4 (aq)">answer
Moles H2SO4 = 0.360 x (37.3/1000) = 1.332 x 10 -2 mol H2SO4 in 25cm3 Moles of NH3 = 2 x 1.332 x 10 -2 = 2.664 x 10 -2 mol in 25cm3 In 250 cm3 = 2.664 x 10 - 1 mol NH3 Mass NH3 = 0.2664 x 17 = 4.57g % mass = (4.57 / 25.37) x 100 = 18.0%
question
Hydrogen sulphide burns in oxygen according to the following equation 2H2S(g) + 3O2(g) --> 2H2O(g) + 2SO2(g) If 4dm3 of H2S are burned in 10 dm3 of oxygen, what is the final volume of the mixture? (assume that all volumes are measured at the same temperature and pressure).
answer
4dm3 H2S needs 6dm3 of O2 Therefore O2 is in excess. H2S = limiting reagent All H2S react to produce 4dm3 H2O and 4dm3 SO2 8 dm3 of products plus 10 -6 = 4 dm3 of O2 left over So 12 dm3 of final mixture
question
An organic compound has the composition by mass of 83.5% carbon, 6.4% hydrogen and 10.1% oxygen. The molar mass of the compound is 158 g mol-1. Determine the molecular formula of the compound.

answer
Carbon 83.5 / 12 = 6.96 Hydrogen 6.4 / 1 = 6.4 Oxygen 10.1 / 16 = 0.63 There froe divide all answers by 0.63 Carbon = 6.96 / 0.63 = 11 Hydrogen = 6.4 / 0.63 = 10 Oxygen = 0.63 / 0.63 = 1
question Cu + H2O" alt="A 25.0 g sample of an oxide of copper, when heated in a stream of hydrogen, forms 3.15 g of water. Find the percentage of copper by mass in the compound. Hint: The Hydrogen removes the copper from the water. CuO + H2 --> Cu + H2O">
Cu + H2O" alt="A 25.0 g sample of an oxide of copper, when heated in a stream of hydrogen, forms 3.15 g of water. Find the percentage of copper by mass in the compound. Hint: The Hydrogen removes the copper from the water. CuO + H2 --> Cu + H2O">
A 25.0 g sample of an oxide of copper, when heated in a stream of hydrogen, forms 3.15 g of water. Find the percentage of copper by mass in the compound. Hint: The Hydrogen removes the copper from the water. CuO + H2 --> Cu + H2O
 Cu + H2O" alt="A 25.0 g sample of an oxide of copper, when heated in a stream of hydrogen, forms 3.15 g of water. Find the percentage of copper by mass in the compound. Hint: The Hydrogen removes the copper from the water. CuO + H2 --> Cu + H2O">
Cu + H2O" alt="A 25.0 g sample of an oxide of copper, when heated in a stream of hydrogen, forms 3.15 g of water. Find the percentage of copper by mass in the compound. Hint: The Hydrogen removes the copper from the water. CuO + H2 --> Cu + H2O">answer
Moles H2O = 3.15 / 18 = 0.175 mol This also = 0.175 mol O in compound Mass of O = 0.175 x 16 = 2.8g Therefore, mass of Cu in compound = 25.0 = 2.8 = 22.2g % by mass = (22.8 / 25) x 100 = 88.8%
question
What is an aqueous state?
answer
A state of matter which applies only to solutions.
question
What is the unit for the concentration and the formula for that?

answer
moldm⁻³ Number of moles (n)/Volume (dm³) The mass of solute in a given volume of solution.
question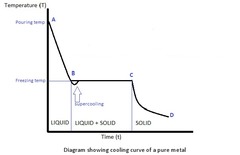
What do the letters represent in the diagram?

answer
A to B: Cooling of liquid B: Beginning of solidification C to D: Plateau(material in the form of solid and liquid phases) D: Solidification ends D to E: Cooling of liquid; solidification is complete; T drops
question
A homogenous mixture

answer
Any mixture that is uniform in composition throughout.



