Physical Science – Atomic Structure – Flashcards
Unlock all answers in this set
Unlock answersquestion
Atomic Structure
answer
The atom consists of three components: Protons, Neutrons, and Electrons
question
Atomic Number

answer
The order of an element in Mendeleev's table of the elements; Often represented by the symbol Z and it dictates the number of protons in the nucleus
question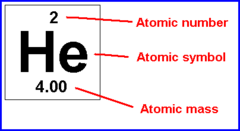
Atomic Symbol

answer
A notation for one of the chemical elements, consisting of letters - First letter is always capitalized and any letters after that is lowercase (For example... Ne, O, C, Mg, etc.)
question
Atomic Mass
answer
The mass of a single atom (Round accordingly and use as Mass Number)
question
Bohr
answer
A Danish physicist (1885-1962); created a new atomic model; described electrons as moving around the nucleus in fixed orbits and having a set amount of energy
question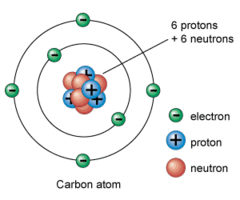
Electron

answer
A subatomic particle in an atom's nucleus that has a negative charge of -1
question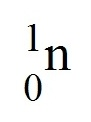
Neutron
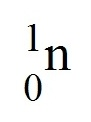
answer
A subatomic particle that has no charge and that is found in the nucleus of an atoma
question
Nucleus
answer
The positively charged dense center of an atom
question
Proton
answer
A subatomic particle in an atom's nucleus that has a positive charge of +1
question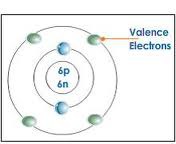
Valence electrons

answer
Electrons on the outermost energy level of an atom
question
Where are protons located in the atom?
answer
In the nucleus
question
Where are electrons located in the atom?
answer
In the electron cloud (flying around the nucleus)
question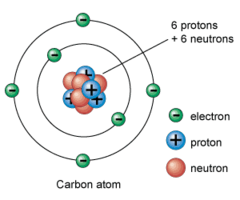
Where are neutrons located in the atom?

answer
In the nucleus
question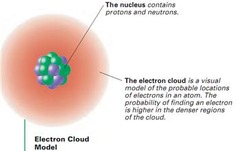
True/False Electrons are small and essentially have no mass, so the electron cloud is mostly empty space

answer
True
question
True/False The nucleus contains almost all of the mass of the atom and is extremely dense
answer
True
question
True/False Protons repel other protons
answer
True
question
True/False Electrons repel other electrons
answer
True
question
True/False Electrons and protons repel each other
answer
False
question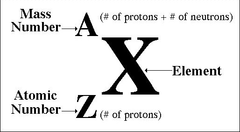
What does the "X" mean in the isotopic symbol?
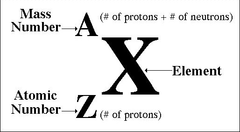
answer
Elemental Symbol
question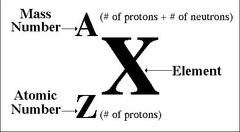
What does the "A" mean in the isotopic symbol?
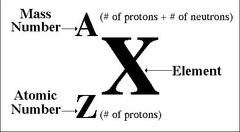
answer
Mass Number
question
What does the "Z" mean in the isotopic symbol?

answer
Atomic Number
question
How do you determine the number of protons in an atom?
answer
Number of Protons = Atomic Number
question
How do you determine the number of electrons in an atom?
answer
Number of Electrons = Number of Protons ONLY IF the atom is neutral
question
How do you determine the number of neutrons in an atom?
answer
Number of Protons = Mass Number - Atomic Number (A - Z)
question
What are the vertical columns in the periodic table called?
answer
Groups
question
What are the horizontal columns in the periodic table called?
answer
Periods
question
Write out the isotopic symbol and give the number of protons, electrons and neutrons in a neutral Helium atom
answer
Number of Protons = 2 Number of Electrons = 2 Number of Neutrons = 4 - 2 = 2
question
Write out the isotopic symbol and give the number of protons, electrons and neutrons in a neutral Fluorine atom
answer
Number of Protons = 9 Number of Electrons = 9 Number of Neutrons = 19 - 9 = 10
question
Write out the isotopic symbol and give the number of protons, electrons and neutrons in a neutral Sodium atom
answer
Number of Protons = 11 Number of Electrons = 11 Number of Neutrons = 23 - 11 = 12
question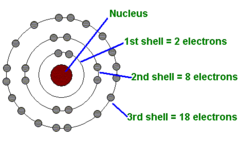
How many electrons can you fit on each shell in a Bohr's model?

answer
1st Shell - 2 2nd Shell - 8 3rd Shell - 8 to 18 4th Shell - 8 to 32



