Part 1 Inorganic Chemistry – Atomic Structure and Periodic Table – Flashcards
Unlock all answers in this set
Unlock answersquestion
Basic format of Atom's mass and atomic number on periodic table A=(?) Z=(?)
answer
A= Mass Number Z= Atomic Number Image is of the written/text format for atoms.
question
Isotopes
answer
Same Atomic Number=same element Vary in Mass - therefore neutrons vary
question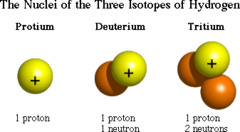
Special Hydrogen Isotope Names

answer
Hydrogen (protium) Deuterium Tritium
question
Ions
answer
Atomic number is the same Charge is different due to addition or subtraction of electrons
question
Common Ions
answer
IA - Na^1+, K^1+ IIA - Mg^2+, Ca^2+, Ba^2+ B Group - Fe^2+ or Fe^3+, Co^2+ or Co^3+, Ni^2+, Zn^2+, Ag^1+ IIIA - Al^3+ VIA - O^2- VIIA - F^1-, Cl^1-, Br^1-, I^1-
question
Common Polyatomic Ions Hydroxide Cyanide Sulfate Phosphate Nitrate Carbonate Bicarbonate Peroxide Permaganate Ammonium Percholorate Chlorate Chlorite HypoChlorite
answer
Hydroxide = OH^1- Cyanide = CN^1- Sulfate = SO4^2- Phosphate = PO4^3- Nitrate = NO3^1- Carbonate = CO3^2- Bicarbonate = HCO3^1- Peroxide = )2^2- Permaganate = MnO4^1- Ammonium = NH4^1+ Percholorate = ClO4^1- Chlorate = ClO3^1- Chlorite = ClO2^1- HypoChlorite =ClO^1-
question
Standard Atomic Weight
answer
Weighted average of all naturally occurring isotopes. so for Nitrogen the std. atomic weight is 14.007 because some most isotopes are 14N then few are 15N
question
Bohr Model
answer
Concept that electron revolves around nucleus of atom
question
Paramagnetic
answer
Has unpaired electrons magnetic field will align the spin and weakly attract the material
question
Diamagnetic
answer
No unpaired electrons and magnetic field will slightly repel the material.
question
Hund's Rule
answer
Orbitals will prefer to have maximum number of half-filled orbitals with parallel spins.
question
Atomic Radii
answer
Increasing protons leads to stronger pull on electron shells --> Decreases Atomic Radii Increasing number of shells leads to increasing Atomic Radii
question
Ionic Radius
answer
Atomic Radii changes when things add or remove electrons to form ions. As electrons are added, the electrons are forced to be near and repel each other making the electron cloud bigger.
question
Ionization Energy (IE)
answer
Energy needed to eject an electron from an atom Outermost electrons are easier to remove (require less energy) that the electrons closest to the nucleus)
question
Electron Affinity (EA)
answer
Change in energy of that occurs when an electron is added also like the Ability or willingness to accept an electron Alkaline Earth Metals low EA as Valence shell is full (2 electrons). Halogens High EA as Valence shell only needs one electron. Nobel gases 0 EA as their shell is already full.
question
Pauling Electronegativity Scale
answer
scale that displays a measure of attraction an atom has towards bonding electrons
question
Anion
answer
Negatively charged Ion often gain an electron to form negative ion. Cl willingly takes an electron making it an anion.
question
Cation
answer
Positive charged ion. Metal elements often become cations, giving up an electron or two



