Organic Chemistry – Chapter 18: Aromatic Compounds – Flashcards
Unlock all answers in this set
Unlock answersquestion
Naming aromatic compounds
answer
For monosubstituted benzenes, benzene is used as the parent name
question
Ortho
answer
Groups are in a 1,2 relationship
question
Meta
answer
Groups are in a 1,3 relationship
question
Para
answer
Groups are in a 1,4 relationship
question
Naming compounds with more than two substituents
answer
Lowest possible numbers are used Listed alphabetically Any of the monosubstituted aromatic compounds can serve as the parent name
question
Stability of benzene
answer
Aromatic rings don't undergo addition reactions
question
Aromaticity
answer
Cyclic, conjugated Unusually stable Planar Undergo substitution that retain conjugation
question
4n+2 rule
answer
A molecule is aromatic only if it is planar, has a monocyclic system of conjugation, and contains 4n+2 pi electrons Molecules with 2,6,10,14,18....are aromatic These numbers fill bonding orbitals
question
Anti-aromatic
answer
Molecules with 4n pi electrons (4,8,12,16) Delocalization of the pi electrons would destabilize the ring
question
Cyclobutadiene example
answer
Four pi electrons Anti-aromatic Highly reactive
question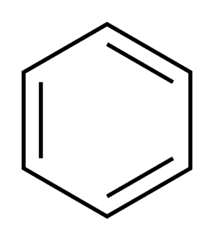
Benzene example

answer
Contains six pi electrons (4n+2) where n = 1 Aromatic
question
Cyclooctatetraene example
answer
Eight pi electrons Not aromatic Electrons localized on four double bonds No cyclic conjugation Not planar (tub-shaped)
question
Frost circles
answer
1. Draw circle 2. Draw a polygon so one point is on the bottom of circle 3. Draw horizontal line at each connection point 4. Draw dotted line through center 5. Identify bonding molecular orbitals (below the line), nonbonding MOs (on the line), and antibonding MOs (above the line)
question
Heterocyclic compounds
answer
Contains two or more different elements in the ring (usually N, O, or S with carbon)
question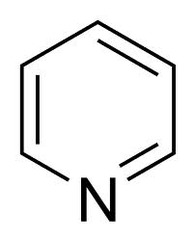
Pyridine

answer
Each carbon contains one pi electron The nitrogen contributes one pi electron The lone pair is not involved in the pi system, as it's in a plane
question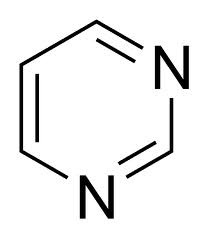
Pyrimidine

answer
Two nitrogen atoms that each contribute a pi electron
question
Pyrrole
answer
Aromatic Six pi electrons Each sp2 orbital contributes one pi electron The lone pair is in a p orbital and so two pi electrons are contributed
question
Imidazole
answer
Both nitrogens are sp2 One N contributes one pi electron The other N contributes two pi electrons from the lone pair, as it is not in a double bond
question
Contributing pi electrons
answer
If the nitrogens are in a double bond, they contribute one pi electron If the nitrogens are not in a double bond, they contribute two pi electrons



