Kaplan- Biochemistry – Flashcards
Unlock all answers in this set
Unlock answersquestion
Most important buffers in physiologic systems
answer
bicarbonate, phosphate and proteins
question
acid
answer
proton donors
question
base
answer
proton acceptors
question
Weak acids
answer
- not complete disassociated - undissociated form (HA) is the conjugate acid and the deprotonated form is the conjugate base
question
Henderson-Hasselbalch equation
answer
determines the amounts of undissociated acid and its conjugated base at any pH based on pKa
question
pKa
answer
- pH where the concentration of the acid and its conjugate base are equal - when the pH and pKa are equal, the concentrations of HA and A- are equal - small changes in pH will bring about large changes in the relative concentrations of HA and A-
question
Titration curve
answer
- at the midpoint half of the acid has been neutralized and HA= A- and pH= pKa
question
Buffering capacity
answer
- buffers are a mixture of weak acids and their conjugate bases - capacity of a buffer to resist change in pH is dependent on two factor: concentration of the buffer and the pH at which it is used - buffer is most effective when it is used in a pH range near the pKa
question
Protein buffering systems
answer
- cytosol of cells contains high concentrations of proteins with amino acid side chains that are weak acids and bases - histidine is the only good amino acid with good buffering capacity at biological pH (imidazole side chain of histidine has a pKa that ranges from 5.6-7.0) - acidic amino acids (glutamic and aspartic acids) and basic amino acids (lysine, histidine and arginine) all contain ionizable side chains
question
Bicarbonate buffering system
answer
- bicarbonate CO2 system is the most important buffer in maintaining the pH of blood plasma and interstitial fluid - carbonic acid is the proton donor - strength in this buffering system lies in the ability of carbonic acid to be converted to carbon dioxide
question
Phosphate buffering system
answer
- intracellular fluids contain high concentrations of inorganic phosphate and many organic phosphate esters that contribute to the buffering power of the cytosol - phosphate buffering system of little importance in the plasma and interstitial fluid - consists of H2PO4- as the proton donor and HPO32-as the proton accepter -
question
Amino acids
answer
- 19 of 20 have central carbon atom attached to a carboxyl group, an amino group, and a hydrogen atom - amino acids differ from one another by their side chain
question
Which amino acid is not like the others and why?
answer
- proline - side chain forms a cyclic structure with the amino group
question
Amino acid classification
answer
- hydrophilic and hydrophobia - hydrophobic AA have side chains with aliphatic groups or aromatic structure - hydrophilic amino acids have side chains that contain O, N or S. Can be positive, negative or neutral
question
Positive amino acid side chains
answer
- Lysine - Arginine - Histidine
question
Negative AA Side chains
answer
- Aspartate - Glutamate
question
Neutral amino acid side chains
answer
- Serine - Threonine - Cysteine - Methionine - Asparagine - Glutamine
question
Hydrophobic amino acids- non polar
answer
- Glycine - Alanine - Valine - Leucine - Isoleucine - Proline
question
Hydrophobic aromatic side chains
answer
- phenylalanine - tyrosine - tryptophan
question
Nonessential vs essential amino acids
answer
- nonessential can by synthesized de novo and essential must be obtained from the digestion of dietary proteins - nonessential amino acids are synthesized from intermediates of glycolysis and the citric acid cycle
question
Cystine
answer
- formed in proteins by the reaction of two cysteine side chains that forms a disulfide linkage - found most frequently in extracellular proteins
question
Hydroxyproline
answer
- formed in an oxygen dependent hydroxylation reaction that occurs in fibroblasts - found in collagen and stabilizes the triple helical structure
question
Phosphotyrosine, phosphoserine, and phosphothreonine
answer
- formed by transferring phosphate from ATP to the hydroxl group of serine, tyrosine and threonine - found in many enzymes and proteins where they serve as regulatory signals
question
Stereochemistry
answer
- alpha carbon atom of all amino acids except glycine is linked to four different chemical groups, making the alpha carbon atom an asymmetric center - asymmetric center has two stereoisomers that are mirror images of each other and are designated as D and L amino acids - only L amino acids are incorporated into proteins
question
Zwitterion
answer
at neutral pH, the species has both positive charge and negative charge
question
Isoelectric point
answer
- pH at which a molecule is electrically neutral
question
What is unique about glycine?
answer
- two hydrogen atoms bonded to the alpha carbon
question
Hartnup disease
answer
- transport protein effect with increased excretion of neutral amino acids - symptoms similar to pellagra - autosomal recessive
question
Phenylketonuria
answer
- phenylalanine hydroxylase or dihydrobiopterin reductase deficiency - buildup of phenylalanine - tyrosine becomes essential - musty body odor, mental retardation, microcephaly - autosomal recessive - treat by decreasing phenylalanine in diet and avoid aspartame
question
Homocystinuria
answer
- increase homocysteine in urine - caused by a deficiency in cystathionine synthase - associated with dislocated lens, deep venous thrombosis, atherosclerosis, mental retardation, and Marfan like features
question
Cystinuria
answer
- transport protein defect with increased excretion of lysine, arginine, cystine, and ornithine - excess cystine precipitates as kidney stones
question
Maple syrup urine disease
answer
- branched chain ketoacid dehydrogenase deficiency - branched chain ketoacidosis from infancy - weight loss, lethargy, alternating hypertonia/ hypotonia - maple syrup odor of urine
question
Propionyl CoA carboxylase deficiency
answer
- neonatal metabolic acidosis, hyperammonemia, elevated propionic acid, hydroxypropionic acid and methyl citric acid - poor feeding, vomiting, lethargy, coma
question
Methylmalonyl CoA mutase deficiency
answer
- symptoms similar to propionyl CoA carboxylase deficiency but accumulating metabolites differ (increased methylmalonic acid)
question
Peptide bonds
answer
- amino acids in a protein are linked together in which the a-carboxyl of one amino acid is linked to the a-amino group of another amino acid - has partial double bond characteristic making i planer and rigid
question
Primary structure of protein
answer
- sequence in which the amino acids occur in the polypeptide - sequence is encoded by the DNA
question
Secondary structure
answer
- organized around the polypeptide backbone and is stabilized by large numbers of hydrogen bonds formed between amino hydrogen atom of one peptide bond and the carbonyl oxygen atom of another - alpha helix: coiled configuration - Beta helix- pleated configuration - motifs: simplex combination of a few secondary structure elements
question
Tertiary structure
answer
- overall protein structure - how alpha helix and beta sheet fold with respect to each other - alpha helix is stabilized by INTRAchain hydrogen bonds and the beta sheet is stabilized by the INTERchain hydrogen bonds
question
domain
answer
- fundamental unit of tertiary structure
question
Quaternary structure
answer
- multiple polypeptide chains - how chains fold with respect to each other - each polypeptide is referred to as a subunit
question
Denaturation
answer
- loss of native confirmation that results in a random coil that has little of the biologic activity of the native protein - could be due to heat, extremes in pH, or detergents
question
Oxidoreductases
answer
- oxidation reduction reactions - frequently use coenzymes NAD+, FAD, NADP+ or O2 as electron acceptors - dehydrogenase, oxidase, reductase
question
Transferases
answer
- transfer of a chemical group from a donor to an acceptor - groups transferred include amino, carboxyl, acyl, glycosyl, phosphoric - transaminase, kinase
question
hydrolases
answer
- cleavage of a bond between carbon and some other atom by the addition of water - protease, phosphatase, amylase
question
Lyases
answer
- nonhydrolytic cleavage of carbon-carbon, carbon-sulfur, and some carbon-nitrogen bonds - aldolase, decarboxylase, dehydratase
question
Isomerases
answer
- interconversion of isomers - epimerase, mutase
question
Ligases
answer
- formation of bonds between carbon and oxygen, nitrogen, or sulfur atoms in reactions that require energy - carboxylase, thiokinase
question
Specificity of enzyme catalyzed reactions
answer
- enzymes have active sites composed of a small number of amino acid side chains - side chain come together to form a 3D site on the surface of the enzyme that is complementary to the structure of the substrate
question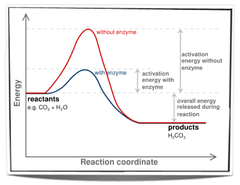
Catalytic properties

answer
- highest part on the curve is the energy of the transition state, an intermediate whose properties resemble both the substrate and the product - Activation energy: delta G or Gts- GS - rate of reaction is inversely proportional to the magnitude of the activation energy - enzymes increase the rate of reaction but have no effect on the equilibrium constant!!
question
Temperature affect on rate of enzyme catalyzed reactions
answer
- rate of most reactions increase ~2x with a 10oC increase in temp - with enzyme catalyzed reactions, there is an optimum temperature beyond which the rate rapidly decreases due to denaturation of the enzyme
question
pH affect on rate of enzyme catalyzed reactions
answer
- optimal activity of most enzymes occurs between pH 5 and 9 - shape of the rate versus pH curve reflects different ionization states for specific amino acid side chains that are required for substrate binding or for catalysis
question
Enzyme concentration on rate of enzyme catalyzed reactions
answer
- rate of an enzyme catalyzed reaction is directly proportional to the concentration of enzyme, provided the substrate is present in concentrations sufficient to saturate the binding sites
question
Substrate concentration on rate of enzyme catalyzed reactions
answer
- at very low concentrations of substrate, first order kinetics are observed with the rate being directly proportional to [S] - when the concentration of substrate is sufficiently high so that all of the binding sites are occupied, zero-order kinetics are seen, with the rate being independent of [S]
question
Michaelis Menten equation
answer
- expression that quantifies the relationship between the rate of an enzyme catalyzed reaction and the substrate concentration - Vmax: rate obtained when all of the enzyme is present as an ES complex, with substrate bound to the active site. Vmax increases as the concentration of enzyme increases - Km: substrate concentration that is required to achieve half of the maximum velocity. Independent of the enzyme concentration
question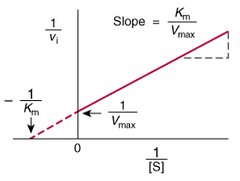
Lineweaver- Burk Plot

answer
- more useful graph for obtaining values of Km and max - obtained by taking the reciprocal of the Michaelis Menten equation
question
Km
answer
- related to the affinity of the enzyme for the substrate - lower the value of Km, the higher the affinity of the enzyme for the substrate - Km is typically equal to the substrate concentration found in the cell
question
Vmax
answer
- efficiency with which substrate is converted to product when all of the active sites are occupied - catalase is highly efficient and RNA polymerase is much less efficient
question
Competitive inhibitors
answer
- structural analogs of the substrate and compete with the substrate for binding to the active site - effects can be overcome by increasing the concentration of substrate - Km for the substrate is increased - Vmax is unchanged - On Lineweaver burk plots the slope is increased by the y intercept stays the same
question
Reversible inhibitors
answer
- alter the kinetic properties of an enzyme by binding noncovalently to the enzyme through multiple interactions with amino acid side chains - effect is removed once its dissociated from the enzyme - three types: competitive, noncompetitive, and uncompetitive
question
Noncompetitive inhibitors
answer
- bind to some site other than the active site and are not structural analogs of the substrate - Vmax of the reaction is decreased - Km remains unchanged - effect cannot be overcome by increasing substrate concentration - on Lineweaver Burk plot, intercept on the y axis is increased and the intercept on the x axis is unchanged
question
Uncompetitive inhibitors
answer
- bind directly to the enzyme substrate complex, but not to the free enzyme - bind causes a conformational change at the active site that renders the enzyme inactive - both max and Km are changed and they both decrease - slope of the line is parallel with the slope of the uninhibited reaction
question
Irreversible inhibitors
answer
- covalently bond to the enzyme, resulting in permanent inactivation of the enzyme - effects of irreversible inhibitors on the kinetic parameters of an enzyme is identical to that of a noncompetitive inhibitor
question
Allosteric regulation
answer
- activity of allosteric enzymes is regulated y the reversible binding of an effector molecule to a site other than the active site - substrate saturation curve for allosteric enzymes are usually sigmoidal - can be positive or negative and act by altering either the Km, Vmax, or both - activators decrease the Km or increase the Vmax - inhibitors increase the Km or decrease the Vmax
question
Covalent modification
answer
- activity of many enzymes is regulated by a phosphorylation/ dephosphorylation cycle in which a specific serine, threonine, or tyrosine side chain becomes modified - phosphorylation can increase or decrease the activity of an enzyme
question
Isoenzymes
answer
- different proteins that catalyze the same reaction but have different catalytic and regulatory properties and frequently differ in tissue and/or organelle specificity - appearance of tissue specific isoenzymes in plasma is of diagnostic value in identifying sites of tissue damage
question
Induction and repression of enzyme synthesis
answer
- because enzyme activity is directly proportional to the amount of enzyme present, one way to regulate enzyme catalyzed reactions is to alter the rate at which enzymes are synthesized - frequently mediated by steroid or thyroid hormones that act in the nucleus to increase or decrease the rate of transcription, and secondarily, protein synthesis
question
Coenzymes
answer
- serves as an intermediate carrier of some specific functional group - small organic molecules that are more stable than proteins - derived from vitamins - vitamins cannot be synthesized de novo by human tissues but can be synthesized by bacteria normally present in the gut - all water soluble vitamins and some fat soluble vitamins serve as precursors for coenzymes
question
Niacin (B3)
answer
- water soluble vitamin - found in whole grains, meat and nuts - niacin is converted to nicotinamide which is then incorporated into the coenzymes NAD+ and NADP+ - these coenzyme are important in lipid and carbohydrate metabolism in which they act as carriers of hydride ions in oxidation and reduction reactions - NAD+ is used in oxidative reactions found in the mitochondria and NADPH is used in reductive pathways found in the cytosol - deficiency may cause pellagra (diarrhea, dementia, dermatitis)
question
Riboflavin
answer
- vitamin B2 - present in organ meat, whole grains and dairy products - coenzymes derived include FMN and FAD both of which act as carriers of hydrogen atoms in oxidation and reduction reactions - coenzymes are important in the oxidation of carbohydrates, lipids and amino acids and are found primarily in the mitochondria - increased riboflavin needed in periods of growth, pregnancy, lactation and wound healing - patients with a deficiency develop lesions of the lips, mouth, skin and genitalia
question
Thiamine
answer
- vitamin B1 - present in meat, beans, peas and grains - coenzyme derived is thiamine pyrophosphate which functions in oxidative decarboxylation of a-ketoacids - enzymes: pyruvate dehydrogenase, a-ketoglutarate dehydrogenase, transketolase - deficiency caused by alcoholism and pregnancy - deficiency can lead to Wernicke, Korsakoff, and high output cardiac failure
question
Pyroxidine
answer
- vitamin B6 - coenzyme derived from this vitamin is pyridoxal phosphate, which acts as a carrier of amino groups in transamination, decarboxylation, racemization, and dehydration reactions - proxidine deficiency may develop during pregnancy or alcoholism , with women on oral contraceptives, and with prolonged exposure to isoniazid or penicillamide therapy - deficit and excess of pyridoxine may lead to peripheral neuropathy and dermatitis
question
Pantothenic acid
answer
- coenzyme derived is coenzyme A which acts as a carrier of acyl groups and is important in lipid metabolism - has a sulfhydryl group that forms a high energy thirster linkage with the carboxyl group of fatty acids - deficiency is rare
question
Biotin
answer
- found in many foods and synthesized in intestinal bacteria - conversion to a coenzyme simply requires that it be covalently linked to the appropriate enzymes - enzymes: - pyruvate carboxylase (gluconeogenesis) - acetyl co a carboxylase (fatty acid synthesis) - propionyl coA carboxylase (branched chain among acid catabolism) - excessive consumption of raw eggs impairs absorption due to avidin - also antibiotics that can alter intestinal flora - symptoms of deficiency include alopecia, skin and bowel inflammation, and muscle pain
question
Folic acid
answer
- present in liver, fresh fruit and leafy greens - coenzyme: tetrahydrofolic acid, which acts as a carrier of one carbon fragments in metabolism at all stage soft oxidation - Pathways: thymidine (pyridimine) synthesis and purine synthesis - deficiency most commonly seen in pregnancy and alcoholism - If deficient, can see megaloblastic anemia, homocystinemia with risk of DVT and atherosclerosis, neural tube defects in fetus (if seen in early pregnancy)
question
Vitamin C
answer
- ascorbic acid found in fruits and veggies - oxidized and reduced form but reduced form is active form - enzymes: prolyl and lysyl hydroxylases, dopamin B-hydroxylase - functions: collagen synthesis, catecholamine synthesis and absorption of iron in GI tract - diet deficient in fruits and veggies can cause deficiency - deficiency causes scurvy
question
Vitamin B12
answer
- cobalamin - synthesized exclusively by microorganisms but is conserved in animal tissues - coenzyme for methylation of homocysteine to methionine and conversion of methylmalonyl-CoA to succinyl-CoA - absorption of B12 requires intrinsic factor which is synthesized by the parietal cells - deficiency leads to megaloblastic anemia -
question
Fat soluble vitamins
answer
- vitamin A - vitamin D - vitamin K - vitamin E
question
Vitamin A
answer
- exists in three forms: retinal, retinol and retinoic acid - patients with fat malabsorption or or celiac disease can become vitamin A deficient and produce night blindness - excess vitamin A is bad too and can cause joint pain, headache and long bone thickening
question
Retinal
answer
- acts as a cofactor for protein opsin to form a rhodopsin complex, which acts as a light receptor in the visual process
question
retinol and retinoic acid
answer
- required for growth, differentiation, and maintenance of epithelial cells - bind to nuclear receptors and regulate the rate of transcription for specific genes
question
Vitamin D
answer
- found in fish oils, liver and milk - can be synthesized in human skin by UV radiation - activated by sequential hydroxylation in the liver and kidney to produce the active form of the vitamin 1,25- (OH)2- VitD - increases intestinal calcium and phosphate absorption - binds to nuclear receptors and increases the rate of transcription - also acts with PTH to mobilize calcium from the bone - deficiency can come from lack of sunshine or renal failure and leads to rickets in children and osteomalacia in adults
question
Vitamin K
answer
- synthesized by intestinal bacteria and supplied by leafy greens - acts as a coenzyme of glutamate carboxylase- enzyme that works in several of the clotting factors - deficiency of vitamin K causes an accumulation of preprothrombin, a deficiency in prothrobim and an increase in clotting time - newborns are vitamin K deficient because their intestinal tracts are sterile
question
Vitamin E
answer
- also known as tocopherol - antioxidant - fat malabsorption may lead to deficiency - in newborns, symptoms include hemolytic anemia - in adults, sensory ataxia due to spinocerebellar deregulation may occur
question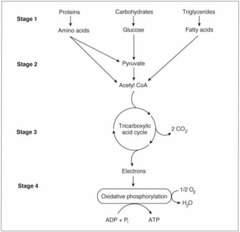
Four stages of extracting energy from food

answer
1- metabolic fuels are hydrolyzed to monomeric building blocks (glucose, amino acids, fatty acids) 2- building blocks are degrade to form acetyl co-A 3- citric acid (Krebs or CTA cycle) oxidize acetyl co-A to CO2 and electron pairs present in carbon-carbon and carbon-hydrogen bonds are transferred to electron carriers (NADH and FADH2) 4- oxidative phosphorylation- energy in the electron pairs of NADH and FADH2 is released via the ETC and synthesized to ATP
question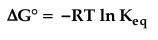
Free energy change (deltaG)
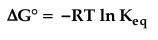
answer
- free energy change is the portion of the total energy that is available for useful work - equal to the difference in energy between the products and the reactants and predicts the direction in which a reaction will proceed spontaneously - standard free energy is constant for any given reaction at "standard conditions" and is shown by the equation - if delta G equals 0, the reaction is at equilibrium
question
exergonic reactions
answer
- delta G is negative and the reaction will proceed spontaneously in the direction written
question
Endergonic reactions
answer
- have a positive deltaG and require the input of energy to proceed in the direction written
question
Coupled reaction system
answer
- endergonic reactions in metabolism are frequently proceeded by being coupled to an exergonic reaction - requirement for a coupled reaction is that the product of the first reaction must be the substrate for the second reaction - cells often couple energetically unfavorable reactions with ATP hydrolysis to force the reaction to proceed
question
Substrate level phosphorylation
answer
- conversion of ADP to ATP by the use of high energy phosphate metabolites is known as substrate level phosphorylation - creatinine phosphate, phosphenolpyruvate and 1,3- bisphosphoglycerate are able to do this - Creatinine phosphate is found in muscle and phosphenolpyruvate and 1,3-bisphosphoglycerate are intermediates in glycolysis
question
High-energy carriers of chemical groups in metabolism
answer
- help reactions to be exergonic and therefore proceed spontaneously
question
Oxidation and reduction
answer
- LEO the lion says GER - loss of electrons= oxidation - gain of electrons= reduction - every oxidation is accompanied by a reduction
question
standard reduction potential, Eo
answer
- constant that describes the tendency of a compound to be reduced - stronger electron donors have a more negative reduction potential
question
relationship between standard reduction potential and standard free energy
answer
- n= number of electrons - F= Faraday constant - delta E= E of electron acceptor - E of electron donor - for an oxidation reduction reaction to be exergonic and to proceed spontaneously, delta E must be a positive value
question
Major electron acceptors
answer
- NAD+ and FAD - NAD+ is the electron acceptor in reactions involving oxidation of hydroxylated carbon atoms. Accepts a hydride ion to form NADH - FAD is the electron acceptor in reactions involving the oxidation of two adjacent carbons resulting in a carbon carbon double bond. Hydrogen atom is removed from each carbon and is transferred to FAD to form FADH2
question
Major electron donors
answer
- NADPH and NADH - NADPH- major source of reducing power for biosynthetic pathways - most of the NADPH is formed and used in extramitochondrial reactions - NADH is generated and used primarily in the mitochondria
question
citric acid cycle
answer
- aka tricarboxylic acid cycle or the Krebs cycle - localized in the mitochondria - only occurs under aerobic conditions - each acetyl co-A generated from pyruvate is used to produce 3 NADH, 1 FADH2, and 1 GTP - both FADH2 and NADH deliver electrons to the ETC to generate energy (11 ATP) by oxidative phosphorylation - complete oxidation of one acetyl cos results in 12 ATP
question
Intermediates in the citric acid cycle used as substrates
answer
Citrate- fatty acid synthesis Oxaloacetate- first intermediate in gluconeogenesis Succinyl CoA- required for the synthesis of heme Oxaloacetate and a-ketoglutarate- substrates for amino acid synthesis
question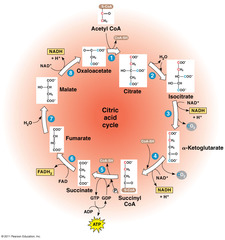
Citric acid cycle

answer
Mneumonic: citric acid is Kreb's starting substrate for mitochondrial oxidation - Citrate, cis-aconitate, isocitrate, a-ketoglutarate, succinyl-CoA, succinate, fumarate, malate, oxaloacetate
question
Key enzymes in the citric acid cycle
answer
- four dehydrogenase (isocitrate, a-ketoglutarate, succinate and malate) all catalyze oxidation reactions - isocitrate dehydrogenase catalyzes one of the rate limiting steps in the citric acid cycle
question
Pyruvate dehydrogeanse: inhibitors and activators
answer
- inhibitors: ATP, acetyl- CoA, NADH - activators: ADP, CoASH, NAD+, Ca2+, insulin
question
citrate synthase: inhibitors and activators
answer
- inhibitor: ATP
question
isocitrate dehydrogenase: inhibitors and activators
answer
- inhibitors: ATP, NADH - activators: ADP
question
a-ketoglutarate: inhibitors and activators
answer
- inhibitors: succinyl co-a, NADH, and ATP
question
Anaplerotic ("filling up") reactions
answer
- when intermediates of the cycle are removed for synthetic purposes, they must be replenished in order to ensure that acetyl-CoA can continue to be oxidized - most importnat reaction for replenishing the cycle is conversion of pyruvate to oxaloacetate, catalyzed by pyruvate carboxylase and requires biotin and bicarbonate as substrate
question
Electron transport chain
answer
- last step in aerobic oxidation - ETC accepts electrons from NADH and FADH2 - energy released at specific steps in the ETC is used to synthesize ATP via oxidative phosphorylation - components of the chain can be separated into four protein-lipid complexes (I,II,III and IV) and two mobile components (CoQ and cyt c)
question
Mitochondrial oxidations
answer
- several substrates (isocitrate, a-ketoglutarate, malate, pyruvate, and glutamate) are oxidized in mitochondria with the formation of NADH - electrons from NADH oxidations enter at complex 1 - succinate, fatty acyl-CoA and a-glycerol phosphate provide electrons in FADH2 through their oxidation - electrons from several FAD-linked dehydrogenase enter at CoQ
question
Shuttles for getting cytoplasmic NADH electrons into the mitochondria
answer
- few oxidative reactions that occur in the cytosol that produce NADH and the mitochondrial membrane is impermeable to NADH - Malate shuttle and a-glycerol phosphate shuttle act as carriers of electrons across the inner mitochondrial membrane - malate shuttle incorporates electrons from the cytosol into NADH - a-glycerol shuttle incorporates cytosol electrons into mitochondrial FADH2
question
Energetics in the ETC
answer
- as electrons move through each complex to the mobile components of the chain, E;0 and G;0 to ensure that electrons flow spontaneously to oxygen
question
Oxidative phosphorylation
answer
- three sites in ETC that energy can be harnessed for oxidative phosphorylation -
question
P/O ratio
answer
- number of ATP molecules produced per O atom reduced - substrates hat are oxidized with the generation of NADH (isocitrate, malate, a-ketoglutarate) have P/O ratios of 3 - substrates that are oxidized with the production of FADH2 (succinate, a-glycerol phosphate) have P/O ratios of 2
question
Efficiency of oxidative phosphylation
answer
- NADH should release sufficient energy to drive the synthesis of 7 moles of ATP - only 3 are produced so the efficiency is approximately only 40% and the remainder of the energy is released as heat
question
Chemiosmotic hypothesis
answer
- when electrons are flowing through the ETC, complexes I, III and IV are pumping protons out of the matrix, creating a proton gradient across the inner mitochondrial membrane - chemiostmotic hypothesis states that the protomotive force associated with the proton gradient drives the synthesis of ATP - movement of protons down the gradient as they re-enter the matrix releases energy that is available for ATP synthesis
question
ATP synthase
answer
- also known as complex V - associated with the inner mitochondrial membrane in close proximity to the electron transport chain - consists of two subunits - F0 subunit spans the membrane and creates a proton channel that allows protons to move BACK into the matrix - F1 subunit protrudes into the matrix and in the presence of a proton gradient catalyzes the condensation of ADP and Pi to form ATP
question
Regulation of the Citric acid cycle and oxidative phosphorylation
answer
- both depend mainly on the availability of O2 and ADP - if O2 is limited, rate of oxidative phosphrlation increases and concentrations of NADH and FADH2 increase - accumulation of NADH inhibits the citric acid cycle - in the presence of O2, the rate depends on the availability of ADP
question
Monosaccharides
answer
- simplest carbohydrates - most are triodes (C3), pentoses (C5) and hexoses (C6) - most importnat monosaccharide is D-glucose
question
aldoses
answer
- monosaccharides that contain an aldehyde
question
ketoses
answer
- monosaccharides containing a keto group
question
Anomeric carbon
answer
- carbonyl carbon - this carbon participates in internal ring structures and glycosidic bonds between monosaccharides
question
Penultimate carbon
answer
- penultimate carbon is the next to last carbon on the chain
question
D and L isomers
answer
- D designation describes the configuration around the penultimate carbon - if the hydroxyl group is on the right, its a D sugar - if the hydroxyl group is on the left, its n L sugar - almost all monosaccharides in the human body are the D-configuraton
question
Epimers
answer
- two monosaccharides are epimers if they differ in the configuration around a single carbon atom - galactose is a 4-epimer of glucose
question
Aldose-ketose isomers
answer
- glucose, an aldose, and fructose, a ketose differ only in the position of the carbonyl group
question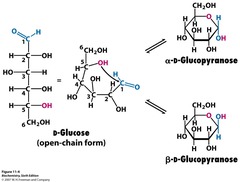
Cyclic structure of monosaccharides

answer
- staring chain structure exists in equilibrium with a ring structure - predominate form of glucose in the body is the B-D-glucose
question
D-gluconic acid
answer
- formed by the oxidation of the aldehyde at C-1, producing an aldonic acid - its a hexose monophosphate shunt
question
D-glucuronic acid
answer
- formed by oxidation of the alcohol at C-6 producing uronic acid - vitamin C (ascorbic acid) is synthesized from glucuronic acid - proteoglycans
question
D-glucosamine
answer
- formed by the substitution of an amino group for the hydroxyl group of C-2 - important structural components of glycoproteins, proteoglycans, and glycolipids
question
Disaccharides
answer
- formed when two monosaccharides are connected by a glycosidic linkage - anomeric carbon of one monosaccharide is usually linked to a hydroxyl group on the second monosaccharide - bond is denoted by a or B depending upon the configuration of the anemic carbon in the linkage
question
Lactose
answer
- milk sugar - disaccharide of galactose linked through its anemic carbon to the 4-hydroxyl group of glucose (B-1,4 glycosidic linkage)
question
Lactase
answer
- found ing the brush border membrane of the small intestine - hydrolyzes lactose to glucose and galactose
question
Sucrose
answer
- table sugar - disaccharide of glucose and fructose linked together through the anomeric carbon atoms in an a-1,2 linkage
question
Sucrase
answer
- hydrolyzes sucrose to glucose and fructose
question
Oligosaccharides
answer
- defined as having between two and 10 monosaccharides linked by glycosidic bonds - found in mucoproteins and glycolipids
question
Polysaccharides
answer
- have more than 10 monosaccharide units - serve as structural components of cels and the ECM, as storage forms for monosaccharides and as dietary fiber - most common polysaccharides are starch, glycogen, cellulose and proteoglycans
question
Starch
answer
- storage form for glucose - composed of amylase and amylopectin
question
Glycogen
answer
- storage form for glucose - major animal polysaccharide - highly branched
question
Cellulose
answer
- linear plant polysaccharide composed of glucose units linked together by B-1,4 glycosidic bonds - not digested by humans because there is no intestinal enzyme for hydrolyzing glucose units linked by B-1,4 glycosidic bonds
question
Proteoglycans
answer
- mucopolysaccharides - major structural components of the ECM - exs: hyaluronic acid, chondroitin sulfate, dermatan sulfate, heparan sulfate, and keratan sulfate - highly asymmetrical and have a high density of negative charge, allowing them to absorb large quantities of water and form viscous solutions - serve as excellent shock absorbers and lubricants
question
glycosaminoglycans
answer
- carbohydrate portions of proteoglycans (GAGs) - contain repeating disaccharides
question
glycoproteins
answer
- contain carbohydrate covalently linked to protein, but there are no repeating disaccharides - found in connective tissue (collagen), in plasma, on cell surfaces as antigens, and as components of mucus - synthesis starts in the endoplasmic reticulum and is completed in the Golgi - last step of glycoprotein synthesis involves putting "zip codes" on proteins that are targeted for a particular destination
question
Amino acid side chains involved in protein-carbohydrate linkage in glycoproteins
answer
- asparagine (N-linkage) is found in plasma and cell surface proteins - serine (O-linkage) is found in mucous and connective tissue proteins - 5- hydroxylysine (O-linkage) is found in collagen
question
How does glucose enter the cell?
answer
- by a group of carrier proteins (GLUT proteins) that span the plasma membrane - net transport across the membrane is ensured by coupling glucose transport with phosphorylation, a process that keeps the intracellular glucose concentration low and continues to shift the equilibrium toward glucose uptake by cells - membrane is highly impermeable to the phosphorylated compounds so the phosphorylation renders glucose transport irreversible
question
GLUT proteins
answer
- responsible for transporting glucose into the cell - differ in tissue specificity, their affinity for glucose, and the maximum rate at which they can transport glucose across the plasma membrane
question
GLUT-4
answer
- skeletal muscle and adipose tissue respond to insulin by increasing their uptake of glucose - these tissues contain GLUT-4
question
GLUT-2
answer
- transporter that has a low affinity for glucose and is not saturated by the increased concentration of glucose the portal circulation following a high carbohydrate meal
question
S-GLUT
answer
- brush border membrane of intestinal and kidney cells contain S-GLUT, a carrier that requires sodium for glucose transport
question
Glucose phosphorylation and trapping
answer
- phosphorylation of glucose in most cells is catalyzed by hexokinase - liver contains glucokinase
question
Glycolysis
answer
- central pathway of glucose metabolism - occurs in the cytosol of all cells - converts glucose to pyruvate and for each mole of glucose converted, 2 moles of ATP are consumed and 4 are generated with a net of 2 moles of ATP produced - under aerobic conditions, pyruvate can be completely oxidized into CO2 and H2O resulting in a total of 36-38 moles of ATP per mole of glucose - under anaerobic conditions, pyruvate is converted to lactate and lactate provides a mechanism for regenerating NAD+ from NADH - generates ATP and provides intermediates that can be used in other pathways - can be divided into two stages
question
Stage 1 of glycolysis
answer
- glucose is converted to fructose- 1,6- P2 through three sequential reactions - two keep enzymes are hexokinase and phosphofructokinase - hexokinase uses ATP to convert glucose to glucose-6-phosphate and then it is isomerize to fructose-6-phosphate -PFK-1 uses ATP to add phosphate to the C-1 of fructose-6-phosphate with the formation of fructuose 1,6, bisphosphate - rate limiting step in glycolysis!!!
question
Stage 2 of glycolysis
answer
- function of second stage is to produce ATP - begins with the cleavage of fructose-1,6- bisphosphate by aldolase into two phosphorylated trioses (dihydroxyacetone phosphate and glyceraldehyde-3-phosphate) - reaction allows both trioses to proceed by a common pathway - two intermediates- 1-3 bisphosphoglycerate and phosphoenolpyruvate have enough energy to drive the synthesis of ATP - three important enzymes in stage 2: glyceraldehye 3-phosphate dehydrogenase, 3-phosphoglycerate kinase, and pyruvate kinase
question
Glyceraldehyde-3-phosphate dehydrogenase
answer
- catalyzes a reversible reaction that occurs in two steps - first step the aldehyde group is oxidized to a carboxylic acid with NAD+ being reduced to NADH - second, inorganic phosphate is covalently linked to the carboxyl group, forming 1,3 bisphosphoglycerate
question
3-Phosphoglycerate kinase
answer
- transfers the high energy phosphate group from 1,3 bisphosphoglycerate to ADP, producing ATP and 3-phosphoglycerate - 3-phosphoglycerate kinase is one of the ATP producing enzymes in glycolysis - Phosphoglycerate mutase moves the phosphate group from carbon-3 to carbon 2, forming 2-phosphoglycerate - Enolase then dehydrates phosphoglycerate to form phosphoenolpyruvate
question
Pyruvate kinase
answer
- catalyzes the last reaction - phosphate group from phosphoenolpyruvate is transferred to ADP with the formation of ATP and pyruvate - reaction is irreversible and is secondary site for regulation of glycolysis
question
Lactate dehydrogenase
answer
- participates in glycolysis only under anaerobic conditions - reduces pyruvate to lactate in a reaction that uses NADH and regenerates NAD+ - when oxygenation is poor (such as in exercising muscle, shock, or cardiopulmonary arrest), both the citric acid cycle and oxidative phosphorylation become relatively inactive and most cellular ATP comes from glycolysis
question
Three steps of regulation in glycolysis
answer
- PFK-1- primary site of regulation, inhibited by ATP and citrate - Pyruvate kinase- inhibited by ATP and acetyl co-a - hexokinase- inhibited by glucose-6-P) -glucokinase- not inhibited by glucose-6-P, induced by insulin
question
Metabolic fates of pyruvate
answer
- can be reversibly converted to lactate (via lactate dehydrogenase) and alanine )via transaminase) - can be carboxylated to oxaloacetate, which can replenish TCA cycle intermediates or be used for gluconeogenesis (pyruvate carboxylase) - also can be converted to acetyl co-a (pyruvate dehydrogenase)
question
Pyruvate Dehydrogenase (PDH)
answer
- multi enzyme complex that converts pyruvate to acetyl Co-A by oxidative decarboxylation - irreversible reaction - enzyme complex is located in the mitochondria and consists of three distinct enzyme activities - five coenzymes are required
question
Three enzyme activities in pyruvate dehydrogenase
answer
- decarboxylase - dihydrolipoyl transacetylase - dihydrolipoyl dehydrogenase
question
Five coenzymes
answer
- thiamine pyrophosphate - lipoid acid - coenzyme A - FAD - NAD+
question
Regulation of pyruvate dehydrogenase
answer
- activity is dependent on the energy state of the cell as reflected by the levels of acetyl-CoA, ATP and NADH - activated by ADP, CoA, and NAD+ - regulation is important to fuel conservation
question
What other enzymes have the same structures similar to pyruvate dehydrogenases?
answer
- a-ketoglutarate dehydrogenase for the TCA cycle - branched-chain keto acid dehydrogenase in amino acid catabolism
question
Anaerobic glycolysis
answer
- each mole of glucose consumed produces 2 moles of lactate and 2 moles of ATP
question
Aerobic glycolysis
answer
- for each mole of glucose consumed, 2 moles each of pyruvate, ATP and NADH are produced - NADH can undergo oxidative phosphorylation, producing either 2 or 3 moles of ATP - produces a net of 36-38 ATP
question
Poisons of glycolysis
answer
- fluoride inhibits enolase by complexing with 2-phosphoglycerate and Mg2
question
Gluconeogenesis
answer
- pathway for de novo synthesis of glucose from C3 and C4 precursors - occurs mainly in the liver and kidney - wants to maintain proper blood glucose levels and to provide glucose for the body - brain, CNS and RBCs are dependent on glucose for all or most of their energy - when fasting persists for more than 12-24 hours, liver glycogen stores are exhausted and gluconeogenesis provides glucose for these tissues - primary precursors are lactate, glycerol, and amino acids -6 ATPs are required
question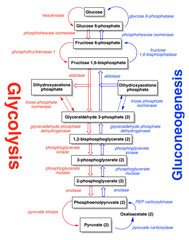
Enzymes in gluconeogenesis

answer
- uses 7 enzymes int he glycolytic pathway that catalyze reversible reactions - also four enzymes unique to gluconeogenesis that are required to bypass the three irreversible reactions in glycolysis
question
Pyruvate carboxylase
answer
- enzyme located in the mitochondria, catalyzes the carbonation of pyruvate to oxaloacetate, the first step in gluconeogenesis - acetyl CoA must be present for the enzyme to function
question
Phosphenolpyruvate carboxykinase (PEPCK)
answer
- catalyzes the second step in gluconeogenesis - cytosolic enzyme that phosphorylates and decarboxylates oxaloacetate to form phosphoenolpyruvate - two enzymes (PEPCK and pyruvate carboxylase) are needed to bypass the irreversible pyruvate kinase reaction
question
Fructose 1,6 bisphosphatase (FBPase-1)
answer
- catalyzes the hydrolysis of fructose-1,6 bisphosphate to fructose-6-phosphate and inorganic phosphate - reaction bypasses the irreversible step of PFK-1 - cytosolic enzyme activated by ATP and citrate
question
Glucose-6-phosphatase (G6Pase)
answer
- catalyzes the last step in gluconeogenesis by removing the phosphate from glucose-6-phsophate and releasing free glucose - bypasses the irreversible hexokinase step in glycolysis - associated with the ER and is found only in the liver, kidney and intestinal epithelium - absence of G6Pase in skeletal muscle accounts for the fact that muscle glycogen cannot serve as a source of blood glucose
question
Precursors for gluconeogenesis
answer
- Lactate- Cori cycle - Alanine- comes from skeletal muscle and is known as the alanine cycle - Glucogenic amino acids- all of the common amino acids except lysine and leucine are gluconeogenic - Glycerol - Odd numbered fatty acids- produces one molecules of propinyl-CoA whicch can be converted to succinyl-CoA, an intermediate in the TCA cycle and then to glucose - Fructose - Galactose
question
Cori cycle
answer
- lactate from RBCs or skeletal muscle is sent to the liver to make glucose that can be returned to RBCs or muscle
question
Regulation of gluconeogenesis
answer
- regulated by substrate availability, enzymatic control and hormonal control
question
Enzymatic control of gluconeogenesis
answer
- pyruvate carboxylase: activated by acetyl-CoA - fructose-1,6- bisphosphatase- activated by ATP and citrate and is inhibited by AMP and fructose-2,6- bisphosphate - glucoes-6-phosphatase- regulated by substrate availability, as glucose-6-phosphate and glucose increase, the activity increases
question
Hormonal control
answer
- main two hormones are glucagon and insulin - glucagon promotes glucose synthesis and release into the blood and insulin promotes glucose uptake and storage - both of these effects are mediated by intracellular concentrations of cAMP
question
Responses to elevated glucagon include
answer
- inhibition of pyruvate kinase (decreases glucose consumption by glycolysis) - decreased concentration of fructose-2,6- bisphosphate - increased synthesis of key enzymes (PEPCK, FBPase-1, and glucose-6-phosphatase) - increased protein degradation - increased lipolysis
question
Glycogen
answer
- highly branched polymer containing glucose molecules linked by a-1.4 glycosiding bonds with a-1,6 glycosidic bonds between the two glucose molecules at the branch points - branching increases the solubility of the molecules and facilitates breakdown of glycogen - glycogens function of the skeletal muscle differs from that of the liver
question
Glycogenin
answer
- initial primer in the synthesis of glycogen and is also the catalyst for the synthesis of the first eight glucose residues of the glycogen molecule
question
Glycogen in skeletal muscle
answer
- skeletal muscle degrades glycogen and rapidly metabolizes glucose via glycolysis - generates ATP required for muscle contraction - thus the function is to provide energy for contraction - in white ("fast") muscle fibers, glucose is released from glycogen and metabolized by glycolysis - in red ("slow") fibers, pyruvate is completely oxidized by the TCA cycle and oxidative phosphorylation
question
Glycogen in the liver
answer
- uses glycogen mainly to regulate blood glucose levels - in hypoglycemic states, glycogen is degraded and glucose is released into he blood - liver glycogen stores become depleted after approximately 12 hours of fasting - in response to hyperglycemia, glucose is removed from the blood and stored in the liver as glycogen
question
Glycogen phosphorylase
answer
- cleaves a-1,4 glycosidic bonds by the addition of inorganic phosphate (phosphorolysis) - rate limiting step in glycogenolysis and is regulated both allosterically and hormonally - will remove glucose units until it gets within 4-5 glucose
question
a[1,4]-->a[1,4] glucan transferase
answer
- debraching enzyme that removes three or four glucose units from a branch point and transfers them to the end of another chain - one a-1,4 bond is being cleaved and another is being formed - elongated chain becomes a substrate for glycogen phosphorylase
question
a-1,6 glucosidase
answer
- debranching enzyme that removes the single glucose unit remaining at the branch point and releases its free glucose
question
Glycogen synthesis
answer
- begins with glucose phosphylation to glucose-6-phosphate with glucokinase int he liver and by hecokinase in other tissues - glucose-6-phosphate is reversibly converted to glucose-1-phosphate by phosphoglucomutase - glucose must be activated or energized before being added to the glycogen polymer and this is done by the formation of UDP-glucose
question
Formation of UDP-glucose
answer
- phosphate group of glucose-1-phosphate reacts with UTP to form UDP-glucose and pyrophosphate (PPi) - reaction is catalyzed by UDP-glucose pyrophosphorylase
question
Elongation of glycogen chains
answer
- glycogen synthase requires an existing glycogen chain serving as a primer - if the chain has completely been degraded then glycogenic serves as the primer - glycogen synthase catalyzes the transfer of glucose from UDP-glucose to the end of a chain and the link created is a a-1,4 glycosidic bond - rate limiting step in glycogen synthesis
question
GLycogenolysis is coupled to the influx of ___ in the cells
answer
- K+ - insulting and glucose are therefore given to treat hyperkalemia (high serum K+), inducing glycogenesis and causing an influx of K+ into the cells
question
a[1,4] a[1,6] glucan transferase
answer
- creates a branch point after approximately 10 glucose units have been added - forming a branch point calls for breaking an a-1,4 linkage and creating a 1,6 linkage
question
Coordinate regulation of glycogenesis and glycogenolysis
answer
- glycogen phosphorylase and glycogen synthase are regulated in a reciprocal manner- when one enzyme is active, the other isn't primary mode of regulation for both enzymes is hormonal and is mediated by phosphorylation/ dephosphorylation
question
Glycogen phosphorylase regulation
answer
- phosphorylation of a specific serine side chain activates the enzyme - activation of a phosphorylase is initiated by the binding of glycogen to liver cell receptors or by the binding of epinephrine to muscle receptors - both of these hormones increase the intracellular synthesis of cAMP, resulting in the activation of a cAMP-dependent protein kinase - cascade system from there and it amplifies the response initiated by the binding of a few hormone molecules and generates millions of glucose-1-phosphate
question
Glycogen synthase
answer
- asme signals that activate glycogen phosphorylase inactivate glycogen synthase - glucagon and epinephrine stimulate the phosphorylation and inactivation of glycogen synthase - inactive form of glycogen synthase in muscle can be allosterically activated by glucose-6-phosphate
question
Glycogen storage disease Type 1: Von Gierke Disease
answer
- decreased glucose-6-phosphate - severe hypoglycemia - lactic acidosis - hepatomegaly - hyperlipidemia - hyperuricemia - short stature
question
Glycogen Storage Disease Type II: Pompe Disease
answer
- decreased lysosomal a-1,4-glucosidase - cardiomegaly - muscle weakness - death by 2 years
question
Glycogen Storage Disease Type III: Cori disease
answer
- decreased glycogen debranching enzyme - mild hypoglycemia - liver enlargement
question
Glycogen Storage Disease Type IV: Andersen disease
answer
- decreased branching enzyme - infantile hypotonia - cirrhosis - death by 2 years
question
Glycogen Storage Disease Type V: McArdle disease
answer
- decreased muscle glycogen phosphorylase - muscle cramps/weakness during initial phase of exercise - possible rhabdomyolysis and myoglobinuria
question
Glycogen Storage Disease Type VI: Hers disease
answer
- decreased hepatic glycogen phosphorylase - mild fasting hypoglycemia - hepatomegaly - cirrhosis
question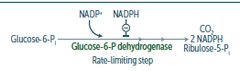
Hexose Monophosphate (HMP) Shunt

answer
- aka pentose phosphate pathway - produces CO2, pentose phsophates, and NADPH - shunt branches off glycolysis at glucose-6-phosphate and reenters at fructose-6-phosphate - in the cytosol - neither produces nor consumes ATP - almost all NADPH required in reductive biosynthetic processes comes from the HMP shunt - synthesis of nucleotides and some coenzymes (NAD+, NADP+, FAD, CoA) require ribose- 5 phosphate, which is supplied y the HMP shunt
question
RBCs and NADPH
answer
- RBCs require large amounts of NADPH to maintain the reduced form of glutathione - reduced glutathione helps prevent hemolysis by neutralizing the effects of strong oxidizing agents such as superoxide and hydrogen peroxide
question
Oxidative phase of the HMP shunt
answer
- produces 2 moles of NADPH per glucose oxidized - consists of three reactions starting with glucose-6-phosphate and resulting in ribulose-5-phosphate - all of these reactions are essentially irreversible
question
Nonoxidative phase of the HMP shunt
answer
- all of these reactions are reversible - Ribulose-5-phosphate is isomerize to ribose-5-phosphate - key enzymes int he transfer reactions are transketolase and transaldolase - major function is to provide a pathway for recycling excess pentoses - important in tissues that require larger amounts of NADPH than pentoses
question
Regulation of the HMP shunt
answer
- rate limiting step in the pathway is the initial reaction catalyzed by glucose-6-phosphate dehydrogenase - amount of this enzyme present in the liver and adipose increases when the diet contains large amounts of carbohydrate - glucose-6-phosphate dehydrogenase is allosterically activated by NADP+ and inhibited by NADPH and palmitoyl-CoA
question
Fructose metabolism
answer
- primary site of fructose metabolism is the liver - liver has three enzymes (fructokinase, aldolase B, and glyceraldehyde kinase) than covert fructose into triodes (intermediates in glycolysis) - enzymes of glycolysis, gluconeogenesis, and glycogenesis also allows dietary fructose to be converted to blood glucose or glycogen
question
Fructose metabolism by the liver
answer
- starts with conversion to fructose-1-phosphate by fructokinase - fructose-1-phsophate is split into two C3 fragments by aldolase B, producing dihydroxyacetone and glyceraldehyde - glyceraldehyde can be phosphorylated to glyceraldehyde-3-phosphate by triosekinase - both dihydroxyacetone and glyceraldehyde-3-phosphate are intermediates in glycolysis
question
Abnormalities in fructose metabolism
answer
- arises from deficiencies in fructokinase and aldolase B - deficiency in fructokinase results in essential fructosuria - a deficiency in aldolase B results in hereditary fructose intolerance - aldolase B cleaves fructose-1-phosphate to glyceraldehyde and dihydroxyacetone phosphate - results in accumulation of fructose-1-phosphate in the liver which inhibits glycogen phosphorylase - both glycogenolysis and gluconeogenesis are impaired resulting in severe hypoglycemia
question
Galactose
answer
- primary source of galactose is milk - metabolism of galactose occurs almost entirely in the liver - three enzymes (glactokinase, gal-1-P:flu-1-P uridyl transferase and UDP-galactose-4-epimerase) are required to assimilate galactose into the central pathways of carbohydrate metabolism
question
Galactose metabolism
answer
- starts by phosphorylation to galactose-1-phosphate by galactokinase - all dietary galactose is phosphorylated in the liver - galactose-1-phosphate is then exchanged for the glucose-1-phosphate moiety of UDP glucose and this reaction is catalyzed by uridyl transferase - results in UDP-galactose and glucose-1-phosphate - finally UDP-galactose is recycled to UDP-glucose by UDP-galactose-4-epimerase
question
Fatty acid
answer
- metabolic fuel - building block for triacylglycerol, phospholipids, and sphingolipids
question
Triacylglycerols
answer
- storage depot and major transport form for fatty acids
question
Cholesterol
answer
- structural component of plasma membrane precursor of bile acids, vitamin D, and steroid hormones
question
Phospholipids
answer
- major building block of membranes - storage site for polyunsaturated fatty acids - signal transduction pathways
question
Sphingolipids
answer
- structural component of membranes - surface antigen
question
Fatty acids
answer
- composed of a long hydrocarbon chain with a carboxyl group at one end - contain both polar and non-polar ends (amphipathic) - two numbering systems: C-numbering system starts at the carboxyl end and the w-numbering system starts at the methyl end
question
Saturated fatty acids
answer
- contain no double bonds - most common saturated fatty acids are palmitic acid and stearic acid
question
Unsaturated fatty acids
answer
- contain one or more double bonds in the cis configuration - monounsaturated fatty acids- contain one double bond. Most common monounsaturated fatty acid is the 18-carbon oleic acid -Polyunsaturated fatty acids contain two or more double bonds
question
Examples of polyunsaturated fatty acids
answer
- Linoleic acid: an 18-carbon w-6 fatty acid with two double bond. Essential fatty acid that must be obtained by dietary sources - Arachidonic acid is a 20 carbon w-6 fatty acid with four double bonds. Can be synthesized by humans from linoleum acid. Becomes essential if linolenic acid is deficient
question
Triacylglycerols (TAG)
answer
- contain a glycerol backbone with three fatty acids link as esters - most common storage form of fatty acids - removal of one fatty acid generates a diglycerol which acts as a second messenger in the phosphatidyllinositol signal transduction pathway
question
Ketone bodies
answer
- soluble metabolic fuel for skeletal muscle, cardiac muscle, kidney and brain - synthesized in the liver from fatty acids and amino acids during prolonged starvation and diabetic ketoacidosis - excreted in the urine - patients with excess ketones present with a fruity breath odor - C4 acids that have a veto or a hydroxyl group attached to the B-carbon atom - two major ketone bodies are acetoacetic acid and B- hydroxybutyric acid
question
Cholesterol and sterol derivatives
answer
- contain a common steroid nucleus- a fused four member ring system that contains 19 carbon atoms - cholesterol, a 27-carbon compound, is the precursor for vitamin D, bile acids, adrenocortical hormones, progesterone, androgens, and estrogens
question
Phospholipids
answer
- amphipathic molecules consisting of two alcohols linked by a phosphodiester bridge - diacylglycerol, alcohol common to all of the phospholipids, contains the non polar structural component - polar head is contributed by the second alcohol and this distinguishes each class of phospholipids
question
Sphingolipids and glycolipids
answer
- contain ceramide as a common structural component - ceramide is composed of sphingosine, a long chain amino alcohol with a saturated fatty acid linked to the amino group - classes of sphingolipids and glycolipids can be differentiated on the basis of the X-group that is esterified to the terminal hydroxyl group of ceramide
question
Lipoprotein
answer
- main vehicle for transporting neutral lipids in the blood - all lipoproteins consist of a hydrophilic shell and a hydrophobic core - hydrophilic shell contains proteins, phospholipids, and unesterified cholesterol - hydrophobic core contains the neutral lipids, triacylglycerols, and cholesterol esters - four major classes chylomicrons, very low density lipoproteins, low-density lipoproteins, and high density lipoproteins
question
Chylomicrons
answer
- least dense of the lipoproteins and do not migrate in an electric field - formed in intestinal mucosa and transport dietary triacylglycerol (TAG) and cholesterol ester (CE) - synthesized in the smooth ER of intestinal epithelial cells resulting in fatty acid release to heart, skeletal muscle, and mammary glands
question
Very low density lipoproteins (VLDL)
answer
- synthesized in the liver and transport TAG and CE - metabolized by lipoprotein lipase to produce remnants and these remnants can be further metabolized to particles of still lower density, or they can be internalized by the liver
question
Low-density lipoproteins (LDL)
answer
- generated from VLDLs and IDLs by the action of lipoprotein lipase thus increasing the relative proportion of cholesterol esters in the neutral core - major function of LDL is to transport cholesterol to extra hepatic tissues
question
High- density lipoproteins (HDL)
answer
- synthesized by the liver and are approximately 50% protein - when the particle is secreted by the liver, the core region is relatively empty - HDLs are circulating reservoir for apoproteins - newly synthesized chylomicrons and VLDL particles obtain some of their apoproteins from HDL reservoirs - reverse chloride transport- HDLs are important important in moving cholesterol from extra hepatic tissues to the liver. Elevated plasma levels of HDL are associated with decreased incidence of coronary atherosclerosis
question
Fatty Acid Metabolism
answer
- six major pathways of fatty acid and lipid metabolism - fatty acids are synthesized from excess carbohydrate in the liver and adipose tissue and are stored as triacylglycerols in adipose tissue when nutrition is sufficient - most of the free fatty acids that are released from adipose tissue are carried by serum albumin to skeletal muscle, cardiac muscle, and the liver
question
Fatty acid synthesis
answer
- fatty acids are synthesized from acetyl coA, bicarbonate, and NADPH - reactions that occur are essentially a reversal of B-oxidation, except for the two processes use different enzymes and occur in different cellular compartments - occurs in the cytosol (B-oxidation occurs in the mitochondria) - humans can synthesize all of the required fatty acids, except linoleic and linolenic acid - acetyl coA must be translocated from the mitochondria, where it is produced, to the cytosol, where it is utilized - because the inner mitochondrial membrane is impermeable to acetyl CoA, the citrate shuttle is used to carry acetyl coA from he mitochondrial matrix to the cytosol
question
Citrate shuttle
answer
- citrate is formed in the mitochondria from oxaloacetate and acetyl coA and then transported into the cytosol - cleaved by citrate lyase to form acetyl co A and oxaloacetate which is converted through two sequential reactions to pyruvate - pyruvate is returned to the mitochondria where it is carboxylated to oxaloacetate to complete the shuttle - citrate shuttle also produces NADPH - about half of the NADPH for fatty acid synthesis is supplied by the pentose phosphate pathway
question
Acetyl-CoA carboxylase
answer
- catalyzes the first step in fatty acid synthesis - requires the presence of both biotin and bicarbonate for converting acetyl-CoA to malonyl-CoA - Malonyl-CoA serves as a donor of two carbon units in the elongation of the fatty acid chain
question
Fatty acid synthase (FAS)
answer
- multi enzyme complex that catalyzes all remaining reactions - lengthens the fatty acyl CoA being synthesized by the sequential addition of two carbon units to the carboxyl end - reduces ketone to full saturation - each cycle adds a two carbon unit and uses two molecules of NADPH to reduce the ketoacyl intermediate - each cycle consists of: - condensation of malonyl-coA to produceB-ketoacylCoA intermediate - reduction of B-ketoacylCoA to B-hydroxyacyl CoA - dehydration of B-hydroxyacyl-CoA to produce only- CoA with a double bond - reduction of the double bond by NADPH to produce a fatty acyl CoA that has been lengthened by two carbons - cycle is repeated until palmitoyl-CoA is formed
question
Elongation and desaturation for fatty acid synthesis
answer
- reactions convert palmitate into a variety of fatty acids that are required for use by the normal cell - these reactions (which occur mainly in the endoplasmic reticulum) rely on malonyl co A for elongation - elongation can also occur in the mitochondria and acetyl coA is used for elongation - desaturation occurs in the endoplasmic reticulum and requires a multi enzyme complex that contains cyt b5 and cyt b5 reductase
question
regulation of fatty acid synthesis
answer
- rate limiting step involves acetyl-CoA carboxylase which catalyzes the first step in the initial reaction converting acetyl-Coa to malonyl-CoA - acetyl-CoA carboxylase is activated by citrate, insulin and a high carb, low fat diet - synthesis of both acetyl-CoA carboxylase and fatty acid synthase complex is induced by insulin
question
Fatty acid oxidation
answer
- most fatty acid oxidation occurs in the mitochondria, although peroxisomes are important in the oxidation of very long chain fatty acids and branched chain fatty acids - uptake and oxidation of fatty acids can be divided into three major stages: activation, transfer of fatty acids into he mitochondria, and B-oxidation of fatty acids
question
Activation
answer
- fatty acids are converted to fatty acyl coA immediately after entering the cell and this traps the fatty acid in the cell - reaction is catalyzed by fatty acyl CoA synthase and is driven by the hydrolysis of pyrophosphate
question
Transfer of fatty acids into the mitochondria
answer
- fatty acids enter the mitochondria via the carnation shuttle (two enzymes and one transporter) - Carnitine acyl transferase-I (CAT-I) is associated with the outer surface of the inner mitochondrial membrane - Carnitine translocase trnasports fatty acyl carnitine into the mitochondria and transports free carnitine back out of the mitochondria - Carnitine-acyl transferase II (CAT-II) is associated with the inner surface of the inner mitochondrial membrane and catalyzes the reformation of fatty acyl coA in the mitochondrial matrix
question
B-oxidation of fatty acids
answer
- occurs in the mitochondria - each cycle involves four reactions and generates one molecules each of acetyl coA, NADH, and FADH2 - oxidizes B-carbon atom - w-oxidation in liver - Activation uses 2 ATP per molecule - carnitine transports fatty acid from the cytosol to the mitochondria - oxidation removes 2 carbons per cycle - 1 acetyl CoA in TCA cycle - 1 FADH2 and 1 NADH --> 5 ATP - oxidation of fatty acids with an odd number of carbon atoms results in the production of one molecule of propionyl CoA which is lateral carbolated to metylmalonyl- CoA - methylmalonyl-CoA +vitamin B12--> succinyl-CoA --> TCA Cycle
question
Four reactions of B-oxidation of fatty acids
answer
- dehydrogenation: fatty acid forms a double bond between the a and B carbons in a reaction that generates FADH2 - Hydration of the double bond produces B-hydroxyacyl-CoA - Dehydrogenation of B-hydroacyl=CoA in an NAD+-dependent reaction generates B-ketoacyl-CoA - Thiolytic cleavage by the addition of CoA-SH to the B-carbon releases acetyl CoA and completes the cycle
question
Energetics of B-oxidation
answer
- net yield of usable energy from the complete oxidation of one molecule of palmitic acid is 129 molecules of ATP
question
Respiratory quotient (RQ)
answer
- moles of CO2 produced divided by moles of O2 consumed - RQ for fatty acid synthesis is 0.7
question
Regulation of fatty acid oxidation
answer
- controlling the rate at which fatty acids enter the mitochondria - CAT-1 is inhibited by malonyl-CoA (first compound committed to fatty acid synthesis) - rate of oxidation is also controlled by the rate at which fatty acids are released from triacylglycerol stores in adipose tissue
question
Ketone body metabolism
answer
- major ketone bodies are acetoacetatic and B-hydroxybutyric acids - these compounds are synthesized from acetyl-CoA by liver mitochondria when excessive amounts of fatty acids are being oxidized and glucose availability is limited - ketone synthesis is inhibited when adequate carbohydrate is available - ketone are used as fuel by extrahepatic tissues
question
Ketosis
answer
- overproduction of ketones - occurs as a result of a high glucagon/ insulin ratio during carbohydrate deficiency states, such as starvation, severe diabetes, and alcoholism - acidic properties of the ketones lower the pH of the plasma causing metabolic acidosis and decreased plasma bicarbonate levels
question
Ketone synthesis
answer
- occurs in the liver mitochondria - condensation of three molecules of acetyl-CoA in two sequential reactions generates B-hydroxy-B-methylglutaryl-CoA (HMG-CoA) - Cleavage of HMG-COA produces acetoacetate and acetyl-CoA - reduction of acetoacetate to B-hydroxybutyrate produces NADH
question
Ketone utilization
answer
- utilization by extra hepatic tissues occurs in the mitochondria - following the uptake of ketones, B-hydroxybuyrate is oxidized back to acetoacetate - conversion of acetoacetate to acetoacetyl-CoA is catalyzed by an enzyme that transfers CoA-SH from succinyl-COA to acetoacetate - absence of this transferase in liver accounts of the inability of the liver to use ketones as fuel
question
Triacylglycerol (TAG) synthesis
answer
- occurs primarily in the liver and adipose tissue - synthesis requires glycerol phosphate which is formed by the reduction of dihydroxyacetone phosphate (DHAP) (glycolysis intermediate) - fatty acids are transferred from fatty acyl-CoA to carbons 1 and 2 of the glycerol backbone by fatty acyl transferase - hydrolysis of the phosphate bond at carbon-3 generates diacylglycerol which is then esterified with a third fatty acid producing triacylglycerol
question
Triacylglycerol hydrolysis
answer
- involves a family of lipases - hormone sensitive lipase is found primarily in adipose tissue where it hydrolyzes the first fatty acid from triacylglycerol- RATE LIMITING step in mobilization of fatty acids from adipose stores - pancreatic lipase- hydrolyzes dietary TAG in the small intestine - lipoprotein lipase (LpL)- produced by the endothelial cells of the vasculature in adipose and muscle tissue and hydrolyzes chylomicrons and VLDL triacylglycerol into free fatty acids and glycerol
question
Cholesterol synthesis
answer
- major site is the liver - enzymes required to synthesize cholesterol are extramitochondrial and are localized in the cytosol or the ER - substrates required are acetyl-CoA, NADPH, ATP and O2 - first step of synthesis is the sequential condensation of three molecules of acetyl-CoA to produce HMG-CoA which is then reduced to mevalonic acid - key enzyme in the overall pathway is HMG-CoA reductase and is the rate limiting step - second stage is conversion of mevalonic acid into activated isoprene units that are designed to condense with each other (IPP and DPP) - third stage, squalene is formed by condensation reactions that use six activated isoprene units - fourth and final stage involves the cyclinization of squalene to form lanosterol which is converted to cholesterol
question
Cholesterol ester hydrolysis
answer
- approximately 70% of the cholesterol found in humans exists as cholesterol ester - most common fatty acid found esterified is oleic acid - pancreatic cholesterol esterase hydrolyzes dietary cholesterol esters in the intestinal lumen. Re-esterification occurs after diffusion into the intestinal mucosal cell but before incorporation into chylomicrons - intracellular cholesterol esterase are found in all tissues but are in highest concentrations in the liver and steroid hormone producing glands
question
Synthesis of cholesterol esters
answer
- Lecithin cholesterol acyltransferase (LCAT)- found in the plasma and participates in reverse cholesterol transport by HDL - Acyl Cholesterol acyltransferaes (ACAT)- found inside the cell and is involved in cholesterol storage - think LCAT is for leaving and ACAT is for accumulation
question
Regulation of cholesterol synthesis
answer
- diets high in fat and carbohydrate stimulate HMG-CoA reductase, the rate limiting enzyme in the pathway - activity is suppressed by high dietary cholesterol and by fasting - HMG-CoA is under hormonal control- stimulated by insulin and thyroxine and inhibited by glucagon
question
Bile acids
answer
- 24 carbon compounds derived from cholesterol - synthesis occurs int he liver and involves four reactions - major excreted form of cholesterol - side chain cleavage of cholesterol results in the C24 compound - conjugation of the double bond carboxyl group ensures complete ionization at physiologic pH - reduction of the double bond in the ring system - hydroxylation at carbons 7 and 12
question
Steroid hormones
answer
- derived from cholesterol in the adrenal cortex, the ovaries, and the testes - initial reaction in the synthesis of all steroid hormones is cleavage of the side chain to generate pregnenolone- RATE LIMITING STEP - pregnenolone is converted to progesterone by 3-B-hydroxysteroid dehydrogenase - 20-22 demolase is located in the mitochondria and its activity is increased in response to hormone binding to membrane receptors - for cells that synthesize glucocorticoids, ACTH stimulates descales; in glucocorticoid synthesis, ACTH stimulates aldolase activity; in mineralocorticoid synthesis, the activity is stimulated by angiotensin II; in androgen and estrogen synthesis, the activity is stimulated by LH
question
Hydroxylation reactions
answer
- a tissue specific synthetic reaction - occur at various positions at the steroid nucleus
question
Side chain cleavage by 17-20 desmolase
answer
- tissue specific synthetic reaction - removes the remainder of the side chain and converts the steroid to a C19 androgen
question
5-a-reductase
answer
- tissue specific synthetic reaction - reduces the double bond in testosterone to form Dihydrotestosterone (DHT) - enzyme is present in tissues that use DHT as the major androgen
question
Aromatase
answer
- tissue specific synthetic reaction - removes the methyl group that extends up between the A and B rings of the steroid nucleus and makes the A ring aromatic - present in tissues that convert androgens to estrogens
question
Phospholipids
answer
- major building blocks of membranes and participate in signal transduction pathways and servee as reservoirs for polyunsaturated fatty acids needed for eicosanoid synthesis
question
Phospholipid synthesis
answer
- ATP phosphorylates ethanolamine - CTP activates phoshpethanolamine - CDP- ethanolamine combines with diacylglycerol to form phosphatidylethanolamine
question
Sphingolypids and glycolipids
answer
- group is classified into five major categories and they all contain ceramide - sphinogmyelin - cerebrosides - sulfatides - globosides - gangliosides - all classes by sphingomyelin are considered glycolipids because of the presence of carbohydrate
question
Sphingomyelin
answer
- major component of membranes of the CNS - only sphinolipid that contains phosphate and the transfer of phosphocholine from UDP-choline to ceramide forms sphingomyelin
question
Cerebrosides and sulfatides
answer
- cerebrosides contain glucose or galactose - addition of sulfate to galactocerebroside generates a sulfatide
question
Globosides
answer
- addition of two or more sugars to ceramide results in globosides - important constitutes of RBC membranes
question
gangliosides
answer
- glycolipids containing neuraminic acid - lipids are found in high concentration in ganglion cells of the CNS
question
synthesis of sphingolipids and glycolipids
answer
- first step is synthesis of the ceramic core - ceramide core is synthesized from three precursors: Palmitoyl-CoA, serine and a long chain fatty acyl-CoA - all classes are formed by the transfer of groups from their activated carriers to a hydroxyl group on the terminal carbon of ceramide
question
Gaucher Disease
answer
- autosomal recessive disorder - deficiency of B-glucocerebrosidase - glucocerebrosides in brain, liver, spleen, bone marrow - has hepatosplenomegaly, neurologic deficits, and mental retardation
question
Fabry disease
answer
- x linked recessive - deficiency of a-galactosidase - leads to renal failure, talangiectasias, skin rash and pain in lower extremities
question
Tay-Sachs disease
answer
- Autosomal recessive - deficiency of hexosaminidase A, Gm2 gangliosides - mental retardation, blindness, muscular weakness, cherry-red spot on macula, death by age 3, common in Jews
question
Niemann-Pick disease
answer
- autosomal recessive - deficiency of sphingomyelinase, sphingomyelin and cholesterol in reticuloendothelial tissues - mental retardation, hepatosplenomegaly, neurologic deficits, foam cells in bone marrow, cherry red spot, death by 3
question
Farber disease
answer
- deficiency in ceramidase - hoarseness, dematitis, skeletal deformations, mental retardation, hepatomegaly
question
Eicosanoids
answer
- prostaglandins, thromboxanes, and leukotrienes - derived from arachiodonic acid - bind to cell surface receptors and act as autocrat and paracrine hormones
question
Prostaglandins
answer
- Prostacyclin, PGI2, is the major prostaglandin and is synthesized by endothelial cells - promotes vasodilation of the coronary arteries and antagonizes platelet aggreation
question
Thromboxanes
answer
- thromboxane A2 is the major thromboxane and it antagonizes prostacyclin leading to platelet aggregation and vasoconstriction - synthesized primarily by platelets
question
Leukotrienes
answer
- play a major role in inflammatory responses and chemotaxis - slow-reacting substance of anaphylaxis (SRS-A) is a combination of leukotrienes that act as a potent smooth muscle constrictor
question
Synthesis of eicosanoids
answer
- Arachiodonic acid si the major precursor and is store din membrane phospholipids - release of AA from the membrane is catalyzed by the action of phospholipase A2
question
Phospholipase A2
answer
- release arachidonic acid from membrane stores, inhibited by glucocorticoids
question
Cyclooxygenase
answer
- catalyzes initial step in the pathway that converts arachidonic acid to prostaglandins and thromboxanes - inhibited by aspirin and NSAIDs
question
Lipoxygenase
answer
- catalyzes the third step in the pathway that converts arachidonic acid to leukotrienes
question
Essential amino acids
answer
- Arginine - Histidine - Isoleucine - Leucine - Lysine - Methionine - Phenylalanine - threonine - tryptophan - valine - Arginine and histidine are essential only in infants and children - Mneumonic: Any help in learning these little molecules proves truly valuable
question
Amino acid pool
answer
- 50% is glutamine and alanine - 10% of the pool is essential amino acids - major drain on the pool is protein synthesis
question
Digestion of dietary protein
answer
- protein digestions starts in the stomach by acidic gastric juice and pepsin - further degradation occurs in the small intestine by the pancreatic proteases - these enzymes (trypsin, chymotrypsin, elastase, carboxypeptidase) are secreted as inactive precursors and are activated in the small intestine by enteropeptidase - enteropeptidase converts trypsinogen to trypsin and trypsin activates the other pancreatic proteases
question
Absorption of amino acids
answer
- transport systems for amino acids in the intestinal cells are similar to those for glucose, where transport across the luminal membrane is NA+- dependent and transport across the contraluminal membrane is Na+- independent
question
Harnup disease
answer
- rare autosomal recessive disorder characterized by the excretion of large quantities of neutral amino acids in the urine- arise from faulty transport mechanisms
question
Cystinuria
answer
- one of the most common amino acidurias - autosomal recessive disease caused by a defect in the transport protein for lysine, arginine, cystine, and ornithine, resulting in their excretion in the urine - results from an inability to reabsorb COAL (cysteine, ornithine, arginine, and lysine) in the renal tubules
question
Amino acid degradation: disposal of a-amino groups
answer
- degradation of amino acids usually begins with removal of the a-amino group - 85% of the amino nitrogen gets excreted in the urine - transaminations and oxidative deamination of glutamate are important in the flow of nitrogen from amino acids to urea
question
Transaminases
answer
- family of enzymes that transfer that a-amino group from an amino acid to an acceptor (a-keto acid) - specific for the amino acid but are not specific for the receptor - most transaminases use a-ketoglutarate as an acceptor, providing a mechanism for funneling the amino groups from many amino acids into a common pool of glutamate - two routes for disposal of the a-amino group glutamate: may be released as ammonia or transferred to oxaloacetate
question
Oxidative deamination
answer
- catalyzed by glutamate dehydrogenase - releases an ammonium ion from glutamate, making it available for urea synthesis
question
transamination to oxaloacetate
answer
- some of the a-amino groups collected in glutamate are transferred to oxaloacetate to form aspartate - aspartate acts as a donor of amino groups in the synthesis of urea - reaction occurs in the cytosol of the liver and is catalyzed by aspartate transaminase (AST)
question
Urea synthesis
answer
- occurs only in the liver - contains two amino groups that are linked by a carboxyl group - one amino group comes from an ammonium ion and the other is donated by aspartate - carbonyl group comes from the bicarbonate
question
Urea cycle
answer
- first two steps occur in the mitochondria and the remaining occur in the cytosol
question
Carbomoyl phosphate synthetase (CPS-I)
answer
- catalyzes the condensation of an ammonium ion and bicarbonate to form cabamoyl phosphate - needs 2 moles of ATP and is the rate limiting step in urea synthesis
question
Ornithine transcarbamoylase (OTCase)
answer
- transfers the carbomyl group from carbamoyl phosphate to ornithine, resulting in citrulline - citrulline is then transported across the inner mitrochodnrial membrane into the cytosol where the remaining reactions occur
question
Argininosuccinate synthetase
answer
- condenses citrulline with aspartate to form arininosuccinate
question
Argininosuccinate lyase
answer
- cleaves argininosuccinate to form arginine and fumarate
question
Arginase
answer
- releases urea from the side chain of arginine - other product is ornithine which is transported back into the mitochondria for participation in another cycle of urea synthesis - arginase is found only in the brain, liver and kidney
question
Alanine
answer
- skeletal muscle uses alanine as a carrier for transporting a-amino groups to the liver, where urea synthesis occurs - amino groups that have been collected in glutamate are subsequently transferred to pyruvate with the formation of alanine - this is catalyzed by alanine aminotransferase - alanine is taken up by the liver where the reverse reaction occurs and this allows amino groups from skeletal muscle to be transferred to the hepatic pool of glutamate
question
Glutamine
answer
- all tissues produce some free ammonia that can be detoxified by the formation of glutamine - importnat in extrahepatic tissues where urea synthesis does not occur and is of particular importance in the brain - skeletal muscle and brain release large amounts of glutamine into the circulation with most of the glutamine being taken up by the kidney and the liver - nitrogen is released from glutamine as ammonium ions but glutaminase and glutamate dehydrogenase
question
Disposal of carbon skeletons
answer
- carbon skeletons are the remnants of amino acids after the amino groups have been removed - carbon skeletons can be oxidized to CO2 and H2O with the generation of energy or they can be used to synthesize glucose or ketones - all of the amino acids can be degraded to seven common metabolites (acetyl-coA, acetoacetyl-coA, pyruvate, oxaloacetate, fumarate, succinyl-CoA, a-ketoglutarate and propionyl-Co) - strictly ketogenic: leu, lys - both ketogenic and glycogenic: lie, he, try, trp - strictly glycogenic: everything else
question
Degradation of branched chain amino acids
answer
- degradation of the branched chain amino acids starts in skeletal muscle where transaminase activity is high - branched chain amino acid transaminase transfers the amino group to a-ketoglutarate to from glutamate and a-keto acids - a-keto acids are decarboxylated by a single branched chain ketoacid dehydrogenase (BCKA-DH) resulting in branched chain acyl-CoA analogs - products of BCKA-DH reaction proceed along different pathways - Val --> Succinyl-CoA - Ile --> Propionyl-CoA, Acetyl-CoA - Leu --> Acetylacyl-CoA
question
BCKA-DH (branched chain ketoacid dehydrogenase)
answer
- multi enzyme complex structurally homologous to pyruvate dehydrogenase and a-ketoglutarate dehydrogenase - all catalyze the oxidative decarboxylation of a-keotacids - composed of three enzymes that require the actions of five coenzymes: NAD+, FAD, CoA, thiamin-pp, and lipoid acid) - located in the mitochondria of most cells
question
Assimilation of Propionyl-CoA into the TCA cycle
answer
- propionyl-CoA results from the degradation of isoleucine and methionine - converted to succinyl-CoA before incorporation in the TCA cycle - deficiency in either propionyl-CoA carboxylase or methylmalonyl-CoA mutters results in organic aciduria
question
Synthesis of nonessential amino acids
answer
- synthesized from intermediates in glycolysis or the TCA cycle, from essential amino acids, or from interrogate pathways
question
Synthesis from intermediates in glycolysis or the TCA cycle
answer
- eight of the non-essential amino acids can be synthesized this way - pyruvate --> alanine - phosphoglycerate -->serine and glycine - a-ketoglutarate --> glutamate - oxalocetate --> aspartate - aspartate ---> asparagine - glutamate --> glutamine
question
synthesis from essential amino acids
answer
- phenylalanine is the precursor for tyrosine and methionine provides the sulfur for cysteine synthesis
question
Phenylalanine hydroxylase
answer
- converts phenylalanine to tyrosine - requires O2 and tetrahjydrobiopterin (THB) - PKU results from a deficiency in phenylalanine hydroxyls or dihydrobiopterin reductase - if there is a build up of phenylalanine, tyrosine becomes an essential amino acid - comes with a musty body odor and mental retardation
question
Cysteine formation
answer
- serine and methionine are required for the synthesis of cysteine - sulfar atom in cysteine originates in dietary methionine and the remainder of the cysteine molecule is derived from serine
question
Methionine synthase
answer
- converts homocysteine back to methionine - only enzyme in human biochemistry known to use tetrahydrofolate (THF) as a methylating agent - one of the two reactions that requires B12
question
Homocystinuria
answer
- excretion of large amounts of homocystine in the urine - most frequently seen in children who present with failure to thrive and visual problems due to displacement of the lens - may be acquired or inherited - deficiency of cystathionine synthase that converts homocysteine to cystathionine
question
Cystathioninuria
answer
- deficiency in pyridoxine (vitamin B6) or from a genetic defect in cystathionase - large amounts of cystathionine around found in the urine and blood
question
Interorgan synthesis of arginine
answer
- synthesis of arginine in humans is dependent on cooperation between the intestinal mucosa and the kidney - first two enzymes are found in intestinal cells and the third and fourth are found in the kidney - only the liver has all of the enzymes - intestinal cell synthesize citrulline and release it into blood - citrulline is extracted by the kidney and converted to arginine - kidney has low levels of organs activity and thus arginine accumulates
question
S- adenosylmethionine (SAM)
answer
- specialized product derived from amino acids - almost all methylation reactions require SAM as a methylating agent - synthesized by the condensation of methionine and ATP
question
Histamine
answer
- specialized product derived from amino acid - decarboxylation of histidine results in histamine which is an important mediator of the inflammatory response - synthesized in mast cells of connective tissue near blood vessels
question
y-Aminobutyric acid (GABA)
answer
- specialized product derived from amino acids - decarboxylation of glutamate results in GABA, an important inhibitory neurotransmitter
question
Serotonin
answer
- specialized product derived from amino acids - hydroxylation of the aromatic ring of tryptophan, follow by its decarboxylation, results in serotonin, a potent vasoconstrictor and stimulator of smooth muscle contraction
question
Catecholamines
answer
- specialized product derived from tyrosine - act as neurotransmitters when synthesized by the brain and act as hormones when produced by the adrenal medulla
question
Melanins
answer
- specialized product derived from tyrosine - pigments of skin and hair - initial reaction is catalyzed by tyrosine hydroxylase - some forms of albinism results from a deficiency in tyrosine hydroxylase
question
Thryoxine
answer
- specialized product derived from tyrosine - synthesis occurs in the follicle cell of the thyroid - synthesis requires tyrosine rick thryglobulin and the uptake of iodine
question
Nitrous oxide
answer
- specialized product derived from arginine in endothelial cells of small blood vessels - NO diffuses not the smooth muscle cells, where its direct effect is to stimulate cyclic GMP synthesis - NO is also an important excitatory molecule in the nervous system - synthesized from arginine by the enzyme nitric oxide synthase
question
Creatine synthesis
answer
- requires arginine, glycine, and S-adenosylmethionine - creatine phosphate is a storage form for high energy phosphate in the muscle - can be used to rapidly regenerate ATP from ADP by the enzyme creatine phosphokinase (CPK) - under normal conditions, amount of creatinine excreted is constant and directly proportional to muscle mass - blood levels are used to clinically assess kidney function or estimate muscle mass
question
Heme synthesis

answer
- requires the assembly of the porphyrin ring, which is derived entirely from glycine and succinyl-CoA - initial step is catalyzed by Delta- ALA synthase (an enzyme that requires pyridoxal phosphate). This is the rate limiting step - 2 moles of delta-ALA produces a prophobilinogen, the first intermediate with a pyrole ring - condensation of four molecules of porphobiligen gives a linear pyrrole which can be cyclized to produce a porphyrinogen - immediate precursor to heme is protoporphyrin IX - insertion of iron by the enzyme heme synthase, also known as ferrochetolaste, results in heme
question
Porphyria
answer
- refers to any abnormality in the pathway of heme synthesis - if the block is early in the pathway, the intermediates that build up are excreted in the urine - if the block is late in the pathway, they are excreted in the urine and feces and accumulate in the skin - lead poisoning can be considered an acquired porphyria because it inhibits ALA dehydrates and heme synthase
question
Heme degradation
answer
- begins in the reticuloendothelial cells where heme oxygenate opens the ring system to give the linear pyrrole biliverdin and carbon monoxide. This is the only reaction in human biochemistry that generates CO - biliverdin is reduced to bilirubin which is further metabolized in the liver - addition of either one or two molecules of glucuronate to bilirubin great increases its solubility - sterocobilin is secreted in the feces and urobilin is secreted in the urine
question
Relationship between storage and transport forms
answer
- fatty acids, glucose, and amino acids serve as the transport forms of triglycerides, glycogen and proteins, respectively - transport forms are found in higher concentrations in the blood - formation of ketone bodies provides an overflow pathway for excess acetyl-CoA
question
When acetyl Co-A is being produced faster than it can be used, what is it converted to?
answer
- ketones - ketones can enter the blood and be taken up by other tissues and used as fuels
question
Relationship between transport forms and intracellular metabolites
answer
- inside the cell, each of the fuels is converted to one or more characteristic intracellular metabolites - amino acids --> glycolytic or TCA intermediates - glucose --> pyruvate - fatty acids --> acetyl-CoA
question
Transitions between metabolic fuels
answer
- both carbohydrates and proteins can be converted to fatty acids - carbohydrate can be used for the synthesis of the nonessential amino acids and protein - acetyl coA cannot be converted to pyruvate and cannot act as a precursor for carbohydrate or protein synthesis - reaction catalyzed by pyruvate dehydrogenase (pyruvate to acetyl-CoA) is an irreversible reaction
question
Well-fed state
answer
- blood glucose levels rise and stimulates the release of insulin - three major target tissues for insulin are liver, muscle and adipose tissue - insulin promotes glycogen synthesis in the liver and muscle - after glycogen stores are filled, liver converts excess glucose to fatty acids and triglycerides - insulin also promotes triglyceride synthesis in adipose tissue and protein synthesis in muscle - mot of the energy needs of the liver are met by oxidation of excess amino acids
question
Overnight fast
answer
- glucagon and epinephrine levels rise during an overnight fast - these hormones exert their effects on skeletal muscle, adipose tissue and the liver - in the liver, glycogen degradation and release of glucose into the blood is stimulated - hepatic gluconeogenesis is also stimulated - release of amino acids from muscle and fatty acids from adipose tissue - amino acids and fatty acids are taken up by the liver where the amino acids provide the carbon skeletons and the oxidation of fatty acids provides the ATP necessary for gluconeogenesis
question
Prolonged fast
answer
- levels are glucagon and epinephrine are markedly elevated - lipolysis is brisk resulting in excess acetyl-CoA that is being used for ketone synthesis - levels of both lipids and ketones are therefore increased - muscle uses fatty acid as the major fuel - brain adapts to using ketones for some of its energy - shift from glucose to ketones during starvation spares proteins - RBCs and renal medullary cells that have few mitochondria still depend on glucose for their energy
question
Storage
answer
- carobhydrate storage: occurs in skeletal muscle and liver. Muscle glycogen accounts for 2/3 of carb storage - fats are stored in adipose tissue, which comprises 1/4-1/3 of the human body weight - proteins are found in all body tissues. Degradation of more than 1/3 of the protein in the body is incompatible with life
question
True or False: Regardless of which fuel is being used, they are all degraded to acetyl co-A
answer
- True - all are degraded to acetyl-CoA which is oxidized by common pathways to CO2 and H2O
question
True or false: Fasting state promotes fat utilization and promotes carbohydrate and protein utilization
answer
- False: it inhibits carbohydrate and protein utilization - in fasting state, gluconeogenesis, proteolysis, and lipolysis are all activated - accumulation of acetyl co-A inhibits pyruvate dehydrogenase and the ability to use carbohydrate and protein as sources of fuel is decreased
question
Fuels for skeletal muscle
answer
- main fuels are glucose and free fatal acids - post-feeding, glucose is major fuel and excess amino acids are oxidized for energy - in the fasting state, fatty acids and ketones are the major energy - when the muscle is anaerobic due to intense contraction, production of pyruvate by glycolysis may exceed the capacity of the citric acid cycle to carry out terminal oxidation and lactic acid accumulates
question
Fuels for Cardiac muscle
answer
- after a meal- glucose and fatty acids are the major fuel - after a brief period of fasting, fatty acids are used - after a longer fast, ketones are used - cardiac muscle behaves much like skeletal muscle except it has a much higher oxidative capacity and many more mitochondria
question
Fuels for the brain
answer
- glucose is the primary fuel - fatty acids cannot cross the BBB and cannot be used at all - between meals brain relies on blood glucose produced by hepatic glycogenolysis or gluconeogenesis only in prolonged fasts does the brain gain the capacity to use ketones for energy (and even then its only 2/3)
question
Fuels for kidney
answer
- uses virtually all fuels: fatty acids, glucose, lactate, amino acids, citrate, glycerol and ketones - renal cortex relies primarily on fatty acids - renal medulla preferentially uses glucose - during fasting, the rate of gluconeogenesis but he kidney is increased
question
Fuels for Liver
answer
- two major roles of liver in fuel metabolism are to maintain a constant level of blood glucose and to synthesize ketones when excess fatty acids are being oxidized - after a meal, glucose concentration increases and the liver extracts excess glucose and returns the concentration in the blood to normal - glucose is used to replenish glycogen stores and any glucose remaining is converted to acetyl-CoA for fatty acid synthesis - in well-fed state, liver derives most of its energy from the oxidation of excess amino acids - between meals, liver releases glucose into the blood - fasting promotes both glycogen degradation and gluconeogenesis - lactate, glycerol, and amino acids provide carbon skeletons for glucose synthesis
question
Fuels for adipose tissue
answer
- after a meal the elevated insulin stimulates glucose uptake - insulin also stimulates fatty acid release from VLDL and chylomicron triglyceride - insulin suppresses the release of fatty acids from adipose tissue - during fasting, the increase in glucagon and epinephrine activates hormone sensitive lipase in fat cells, allowing fatty acid to be released into circulation
question
What hormone promotes fuel storage?
answer
insulin insulin is produced by the B cells in the islets of Langerhans in the pancreas
question
Hormones promoting fuel mobilization
answer
- glucagon: synthesized n the alpha cells of the pancreas - epinephrine: synthesized in the adrenal medulla - norepinephrine: synthesized in the adrenal medulla - cortisol: synthesized in the adrenal cortex - growth hormone: synthesized in the anterior lobe of the pituitary
question
Hormones acting at the plasma membranes of cells
answer
- binding of hormones by cell-surface receptors initiates a chain of events that results in the phosphorylation of specific intracellular proteins - most of these hormones generate "second messengers" inside the cell that simulate specific protein kinases
question
Hormones that act in the nucleus
answer
- hormone-receptor complex binds to DNA and alters the rate of gene expression - steroid hormones, thyroid hormones, and 1,25 dihyrdroxycalciferol (activated vitamin D) all bind to specific receptors and alter the rate of mRNA synthesis - binding of the hormone-receptor complex to "enhancer" sequences in DNA results in increased mRNA synthesis, whereas binding to "silencer" sequences results in decreased mRNA synthesis
question
Glucagon
answer
- release is stimulated by low blood glucose, elevated levels of glucogenic amino acids, or sympathetic stimulation - primary target for glucagon is the liver - also activates lipolysis and the release of fatty acids from adipose tissue - binding of glucagon to receptors in thee tissues results in the phosphorylation of a number of enzymes by camp-dependent protein kinase
question
Epinephrine and Norepinephrine
answer
- released from the adrenal medulla in response to hypoglycemia - two major types of receptors for these hormones: a and B - if B receptors are occupied, adenylate cyclase is activated and cAMP levels are increased. Glycogenolysis in cardiac and skeletal muscle and lipolysis in adipose tissue are all increased - a receptors occupied on liver cells results in other second messengers and stimulation of other protein kinases resulting in the activation of glycogenolysis and gluconeogenesis
question
Insulin lowers cAMP lvels
answer
- insulin antagonizes the effects of glucagon by mechanisms that are not entirely understood - in the liver, insulin activates cAMP phosphodiesterase, the enzyme that degrades cAMP
question
Hormones that modulate gene expression
answer
- steroid and thyroid hormones exert their effects by altering the rate of mRNA synthesis for specific proteins or sets of proteins - glucagon and insulin can also alter the rate of mRNA synthesis for key regulatory enzymes in glycolysis and gluconeogenesis
question
Cortisol
answer
- functions to increase de novo synthesis of glucose - synthesized and secreted by the adrenal cortex - synthesis is stimulated by adrenocorticotropic hormone, a hormone released by the anterior pituitary in time of stress - cortisol has three target tissues: liver, adipose tissue and skeletal muscle - cortisol results in increased lipolysis in adipose tissue and proteolysis in muscle - synthesis of glucose from amino acid precursors in the liver is increased
question
Thyroid hormones (T3 and T4)
answer
- exert their action in the nucleus by altering the rate of gene expression - increases oxygen consumption, lowers blood cholesterol, causes hyperglycemia, and thermogenesis - receptors for the thyroid hormones are found in virtually are of the cells of the body
question
Glucagon and insulin
answer
- can also alter the rate of gene expression - glycogen alters gene expression via cAMP-dependent phosphorylation of nuclear proteins that interact with the DNA - glucagon increases mRNA synthesis for enzymes that are specific for gluconeogenesis (PEPCK, FBPase-1, and glucose-6-phosphatase) and represses the synthesis for key glycolytic enzymes (glucokinase, PFK-1 and pyruvate kinase) - Increases PEPCK, FBPase-1 and glucose-6-phosphatase - decreases glucokinase, PFK-1, and pyruvate kinase
question
Insulin
answer
- can also alter the rate of gene expression - insulin mechanism is unclear - insulin stimulates the synthesis of mRNA for the key glycolytic enzymes and represses mRNA synthesis for gluconeogenic enzymes - increases glucokinase, PFK-1 and pyruvate kinase - decreases PEPCK, FBPase-1, and glucose-6-phosphatase
question
Nucleotides
answer
- building blocks for nucleic acids - consist of three components: a nitrogenous base, a pentose, and a phosphate group - linkage of nitrogenous base to the pentose generates a nucleoside - addition of a phosphate group to the pentose generates a nucleotide - genetic component carried by the sequence of the nitrogenous bases
question
Purines
answer
- nitrogenous bases that contain two rings, both with nitrogen atoms - adenine (A) and guanine (G) are purines
question
Pyrimidines
answer
- contain a single nitrogen-containing ring - three types of pyrimidines are cytosine (C), thymine (T) and uracil (U)
question
Pentose Sugar
answer
- RNA contains ribose and DNA contains deoxyribose - deoxyribose differs from ribose in that there is a hydrogen atom instead of a hydroxyl group attached to the carbon-2
question
Phosphate groups
answer
- form the linkages between the nucleotides - the phosphate group is esterified to the 5' carbon of the pentose - phosphodiester bonds are formed between adjacent uncle tides
question
Three types of phosphodiester linkages fun in nucleic acids
answer
- 3'-5' phosphodiester linkage- most common linkage and forms the backbone of both DNA and RNA molecules - 2'-5' phosphodiester linkage- involved in RNA splicing - 5'-5' phosphodiester linkage- found in the cap structure are the 5' end of eukaryotic messenger RNA
question
Polarity
answer
- polynucleotides hav epolarity - on one end of the polymer, the ribose has a free 3'-hydroxyl group, and on the other end the ribose has a 5'-phosphate group 5'-p------------>OH 3'
question
Differences between DNA and RNA
answer
- pentose: DNA contains deoxyribose and RNA has ribose - nitrogenous bases: DNA contains A,T,G and C and RNA has A, U, G and C - number of strands: DNA is double stranded and RNA is single stranded - function: DNA is the repository of genetic information while RNA represents "working copies" of the DNA that are used int he transfer of genetic information from DNA sequences to protein sequences
question
Link between nitrogenous base and sugar
answer
- in purine nucleotides and nucleosides, the nitrogen-9 of the ring system is linked to the 1'- carbon of the sugar - in pyrimidine nucleosides and nucleotides, the nitrogen-1 ring is linked to to the 1'- carbon of the sugar
question
Primary sequence vs secondary sequence
answer
- primary structure is the nucleotide sequence - secondary structure is the double helix which can form between any antiparallel and complementary sequences - strands are always oriented in an antiparallel fashion
question
Base pairing
answer
- stabilizes the double stranded structure - G and C - A and T in DNA - A and U in RNA
question
Two stabilizing forces for the double stranded structure
answer
- hydrogen bonding: occurs between base pairs - A-T and A-U pair have two hydrogen bonds - G-C pair has three hydrogen bonds - Base stacking interactions: ring structures of the purines and pyrimidines are stacked over one another in the interior of the double helix structure - hydrophobic interactions between adjacent purine and pyrimidine rings add stability to the double helix
question
B DNA
answer
- most common form of DNA - classic right handed, antiparallel helical structure of DNA - approximately 10.5 bases per turn - two grooves (major and minor)
question
Z DNA
answer
- rare form of DNA that is found in sequences that are rich in C-G and in DNA sequences that have long tracts of alternating purines and pyrimidines - forms a left handed, antiparallel helix
question
A DNA
answer
- form of DNA in which the bases are not perfectly perpendicular to the helical axis, but are tiled about 20 degrees from the axis - anhydrous form of B DNA - is a right-handed, antiparallel helix - found in RNA-DNA hybrids
question
What has closed, circular DNA?
answer
- mitochondria DNA and the DNA of most prokaryotes - some viruses - advantage because it is resistant to exonucleases
question
Supercoiling
answer
- important property of closed circular DNA - important for DNA packaging - can be positive or negative
question
Positive vs negative supercoiling
answer
- negative supercoiling twists the DNA in the opposite direction from the clockwise-turn of the right handed double helix - positive sup coiling adds torsional pressure and allows the DNA to be wound more tightly. DNA is twisted in the same direction as the winding of the double helix - DNA is usually negatively supercoiled, the form required for most biologic reactions
question
Topoisomerases
answer
- enzymes that relieve the tension cause by supercoils in replicating DNA - make transient breaks in the DNA by cutting and resealing phosphodiester bonds in the DNA strands
question
Synthesis of nucleotides
answer
- synthesize by salvage pathways or by the pathway for de-novo synthesis - salvage pathways: nucleosides and the nitrogenous bases that are released by the degradation of nucleic acids are stabilized and reutilized - de novo synthesis: starting materials are more elemental precursors such as amino acids, ammonia, bicarbonate, and ribose-5-phosphate - tetrahydrofolate is an important coenzyme in purine and pyrimidine synthesis - salvage pathways are the major route of synthesis and the de novo pathway is used primarily by rapidly dividing cells
question
Major difference between synthesis of purines and pyrimidines
answer
- purines are assembled which attached to ribose-5'-phosphate - pyrimidine ring is completed before it is attached to ribose-5'-phosphate
question
Degradation of nucleotides
answer
- degradation of nucleotides starts with the removal of the phosphate group and the pentose, yielding free purine and pyrimidine bases - amino groups attached to the ring system are removed as ammonia by the action of adenosine deaminase and guanine deaminase
question
Three cellular functions carried out in the nucleus
answer
- DNA synthesis and repair: nucleus contains all of the enzymes and proteins required for DNA synthesis - RNA synthesis: transcription of DNA into RNA occurs in the nucleus by there distinct RNA polymerases - RNA processing: RNA is synthesized as a precursor that is converted to mature RNA in the nucleus by a number of steps
question
Nuclear envelope
answer
- nucleus is separated from the cytoplasm by a double membrane - outer membrane is continuous with the rough ER and is a part of the endomembranous system - inner and outer membranes form two concentric ring and are separated by a perinuclear compartment
question
Nuclear lamin
answer
- inner surface of the nuclear envelope is lined by an electron-dense layer containing three major proteins - network of proteins plays an important role in the structural organization of the nucleus by providing connections between the inner nuclear membrane and the chromatin
question
Nuclear pores
answer
- inner and outer nuclear membranes are fused together at the pores providing channels of communication between the nucleus and the cytosol - water, ions, amino acids and small proteins can freely pass through the press - pores are associated with proteins that are required for transport of large macromolecular complexes into and out of the nucleus
question
Chromatin
answer
- the chromosomes and chromatin are composed of DNA that is associated with histones, a family of positively charged proteins
question
Nucleolus
answer
- nucleolus is a very dense region containing large amounts of RNA, and it functions as the site of ribosome assembly - nucleolus is associated with the nuclear organizer region (NOR) on the chromosome - NOR of the DNA contains many copies of the genes for ribosomal RNA - periphery of the nucleolus has a granular zone that contains ribosomal precursor particles in various stages of assembly - central region has a fibrillar zone containing ribonuclear protein fibrils
question
Interphase
answer
- period of the cell cycle that precedes mitosis - Can be subdivided into 3 parts (G1, S, and G2)
question
G1 Phase
answer
- gap phase - period of cellular growth that precedes DNA synthesis - lasts approximately 12 hours - lipids, carbohydrates, and proteins are synthesized - muscle and nerve cells have a really long G1 phase and appear to have stopped. Such resting cells are said to be in the G0 phase
question
S phase
answer
- synthesis phase - usually lasts for 6-8 hours and it includes the entire period of DNA synthesis and chromosomal replication - rate of RNA synthesis increases and the cell prepares for mitosis
question
G2 phase
answer
- lasts between 3 and 4 hours and resembles the G1 phase in terms of cellular activity - cell is now tetraploid whereas in the G1 phase it was diploid
question
Mitosis
answer
- nuclear division of the cell that results in the reduction the chromosome complement from he tetraploid to the diploid number found in each daughter nucleus - cytokinesis is the division of cytoplasm that results in the enclosure of each daughter nucleus within a separate cell - divided into six phases
question
Preprophase
answer
- chromosomes condense into recognizable thread-like structures in the nucleus - a pair of centrioles are visible in the cytoplasm
question
Prophase
answer
- two copies of each chromosome are separated length-wise into two single chromosomes called chromatids - main structural component of the mitotic apparatus is the mitotic spindle, which begins to form near the end of prophase - when the centrioles begin to separate, bundles of microtubules assemble between them, creating a spindle - at the end of prophase, the nuclear envelope begins to rupture
question
Metaphase
answer
- nuclear envelope and nucleolus disappear during metaphase - spindle moves into the region formerly occupied by the nucleus - chromosomes move toward the midpoint of the spindle, and each chromosome attaches to bundles of spindle microtubules
question
Early anaphase
answer
- chromatids begin to split longitudinally and start migrating toward the poles of the cell - once the chromatids split, they are referred to as chromosomes
question
Late anaphase
answer
- chromosomes aggregate at the poles and a cleavage furrow begins to form, marking the beginning of cytokinesis
question
Telophase
answer
- nucleolus forms and nuclear envelopes appear around each group of daughter chromosomes - condensed chromatin expands again and begins to reappear - cytoplasm divides by deepening the cleavage furrow until it forms two daughter cells, thus completing cytokinesis
question
** Until anaphase, each chromosome contains two sister chromatids. After anaphase, each chromatid is now a separate chromosome
answer
- Prophase: chromosomes coil, nuclear envelope disappears and spindle apparatus forms - Metaphase: chromosomes align - Anaphase: chromatids separate - Telophase: chromosomes uncoil, nuclear envelope reappears, spindle apparatus disassemble, cell divides in two
question
Meiosis
answer
- type of cell divisions that occurs in sperm and ova and reduces the chromosome number by half - occurs before maturation of the gametes - genetic recombination occurs in meiosis and involves the exchange of segments of chromosomes, resulting in a mixing of allelic linkages into new combinations
question
First meiotic division
answer
- six stages - Meiotic prophase- A, B, and C - Meiotic metaphase I - Meiotic anaphase I - Meiotic telophase I
question
Meiotic prophase- Stage A
answer
- chromosomes condense into visible threads, and pairing of homologous chromosomes occur - pairing is precise, except for the X-Y chromosome combination in males - centromeres of homologous chromosomes do not pair
question
Meiotic prophase- Stage B
answer
- homologous chromosome pairing is complete - four chromatids appear in each pair and this is a tetrad
question
Meiotic prophase- Stage C
answer
- recombination or cross-over stage where the interchange of chromatid segments between two paired homologous chromosomes can occur - point of exchange has an X-like appearance and is called a chiasma - at the chiasma, the chromosome pairs are held together and large segments of genes are exchanged between homologous chromosomes
question
Meiotic metaphase I (stage D)
answer
- paired chromosomes line up on the mitotic spindle - two chromosomes of each homologous pair form connections with microtubules that lead to opposite ends of the cell
question
Meiotic anaphase I (stage E)
answer
- both chromatids migrate toward the same end of the cell
question
Meiotic telophase I (stage F)
answer
- each daughter cell gets one member of each chromosome air for a total of 23 double stranded chromosomes
question
Second meiotic divison
answer
- steps are similar to those in a mitotic division, except no DNA synthesis occurs prior to this division - 23 chromosomes divide at the centromere and each of the newly formed daughter germ cells receives 23 chromatids - each germ cell now has a haploid number of chromosomes and half the amount of DNA of a diploid somatic cell
question
Semiconservative replication
answer
- uses each of the parental strands of DNA as a template for directing synthesis of one new daughter DNA molecule
question
DNA polymerase I
answer
- polymerization of nucleotides is catalyzed by DNA polymerase I - also has the exonuclease activities that function in proofreading and repair mechanisms - requires all four deoxyribonucleotides and Mg2+ for activity - occasionally a polymerases add a nucleotides on the 3' end that cannot hydrogen bond to the template strand. Polymerase stops and removes the unpaired base from the 3' end so that polymerization can begin again
question
All DNA polymerases
answer
- must synthesize 5' to 3' - must use an antiparallel template - require a nucleotide with a free 3'-OH as a primer - are associated with proofreading enzymes
question
DNA polymerase II and III
answer
- function of DNA Polymerase II is unknown - DNA polymerase III is a part of a multi protein complex and is the major replicating enzyme in E. coli
question
DNA ligase
answer
- catalyzes the formation of a phosphodiester bond between the 3'-OH of one fragment of DNA and the 5' monophosphate group of an adjacent DNA fragment - formation of a phosphodiester bond is an endergonic reaction and requires the input of energy that is supplied by NAD+ in bacteria and by ATP in animal cells and viruses
question
Primase
answer
- primer molecule that is required by DNA Polymerase is a short strand of RNA that is complementary to the template strands of DNA - primer is synthesized by a specific RNA polymerase known as primase - primase does not require a primer for invitation of polynucleotide synthesis
question
Helicases
answer
- helicases unwind the two strands in the DNA helix, must occur before DNA replication can begin - requires energy (ATP)
question
Single-strand DNA binding (SSB) proteins
answer
- single strands of DNA resulting from the action of helices are prevented from reannealingby binding to SSB proteins - proteins also protect the single-stranded DNA from cleavage by nucleases
question
DNA topoisomerases, class I
answer
- relax superhelical DNA in front of the replication fork by creating a transient nick in one of the strands - do not require ATP
question
DNA topoisomerases, class II
answer
- introduce negative supercoils in the DNA molecule - requires ATP and involves transient breaking and resealing of both strands of DNA
question
Gyrase
answer
- class II topoisomerase used by E, Coli to promote DNA helix unwinding during replication
question
Unwinding of parental DNA
answer
- at site of replication the two parental strands to not completely separate but are unwound in a localized region known as the replication fork - replication fork can move in either direction from he origin of replication
question
Synthesis of the leading strand
answer
- leading strand is complemental to the parent strand that runs in the 3'-5' direction - synthesized continuously
question
Synthesis of the lagging strand
answer
- synthesis occurs discontinuously producing small fragments known as Okazaki fragments - fragments are later joined by DNA ligase to form a continuous lagging strand
question
RNA Primer
answer
- synthesis of the leading strand and each Okazaki fragment of the lagging strand starts with a short RNA primer - primer is complementary and antiparallel to the 5'-3' parental strand - the 3'-OH of the last ribonucleotide in the primer serves as the shite at which the first deoxyribonucleotide is added by the DNA polymerase
question
Conversion of Okazaki fragments to a continuous strand
answer
- when DNA polymerase III approaches another RNA primer, it dissociates and DNA Polymerase I enters - DNA polymerase I removes the ribonucleotides one at a time from the 5' end then adds complementary deoxyribonucleotides to fill in the gaps - DNA ligase forms a phosphodiester bond between two adjacent DNA fragments
question
Inhibitors of DNA replication
answer
- purine analogs - pryimidine analogs- inhbit thymidylate synthase (critical enzyme for DNA synthesis) - folic acid analogs- act as powerful competitive inhibitors of dihydrofolic acid reductase
question
Mutation
answer
- heritable change in the nucleotide sequence of DNA
question
Mutagen
answer
- chemical that alters the structure of a base in DNA
question
Point Mutation
answer
- change in a single nucleotide - two types: transition and transversion
question
Transition
answer
- change of one purine for another purine or the change of one pyrimidine to another pyrimidine - occurs from misfiring of bases duringDNA replication or from chemical insult
question
Transversion
answer
- exchange of a purine from a pyrimidine and vice versa
question
Nucleotide excision repair mechanism of UV damaged DNA
answer
- UV light induces dimers between adjacent pyrimidines in DNA - four steps: incision, removal, polymerization and ligation - Incision: Uv-specific endonuclease recognizes the damaged DNA and makes a nick in the phosphodiester backbone several bases away on each side of the dimer - Removal: helicase removes the oligonucleotide - Polymerization: DNA polymerase fills in the gap by adding nucleotides, starting with the addition of the first nucleotide to the free 3'-OH group created by the incision and moving in a 5'-3' direction - Ligation: DNA ligase forms a phosphodiester bond between the new DNA segment and the original DNA strand
question
Base excision repair
answer
- most common base transition is deamination of cytosine to uracil in the DNA duplex - three steps: removal of the uracil base, cleavage of phosphodiester, insertion of new cytosine nucleotide and ligation - removal of uracil base: uracil-DNA glycosidase recognizes the mismatch and hydrolyzes the N-glycosidic bond between the uracil and deoxyribose moiety - cleavage of phosphodiester: specific endonuclease recognizes the damage and nicks the phosphodiester bond adjacent to the missing base to release deoxyribose and create a gap - insertion of new cytosine nucleotide and ligation: DNA polymerase fills the gap and the nick is sealed by DNA ligase
question
Diseases associated with DNA repair
answer
- xeroderma pigmentosa, ataxia telangiectasia, Bloom syndrome, and Fanconi anemia - all of these disease occur in high frequency and are associated with a predisposition to malignancy
question
Transcription
answer
- process of making an RNA copy of DNA - catalyzed by the enzyme RNA polymerase - RNA strand is always synthesized in the 5'-3' direction but from an antiparallel and complementary DNA template strand
question
Bacterial promoter region (prokaryote transcription)
answer
- region of DNA that binds RNA polymerase and allows transcription to be initiated - promoter region contains a starting point at which transcription is initiated and two consensus sequences that are recognized by RNA polymerase - sequence at which RNA polymerase binds are located at positions -10 and -35 - starting point is designated as +1
question
Pribnow box (prokaryote transcription)
answer
- an AT rich sequence located 10 base pairs upstream from the site at which transcription begins - present in almost all promoters and is involved in the intimal unwinding of DNA by RNA Polymerase - typically located at -10
question
Hexamer at -35 position (prokaryote transcription)
answer
- another consensus sequence of six nucleotides is located 35 base pairs upstream from the start site for transcription - involved in the initial recognition of the promoter by RNA Polymerase
question
start site (prokaryote transcription)
answer
- first nucleotide transcribed into RNA is usually a purine, A or G - next two nucleotides can be any of the four nucleotides found in RNA
question
Rho-independent termination (prokaryote transcription)
answer
- occurs when the newly synthesized RNA folds back on itself and forms a hairpin loop that is stabilized by hydrogen bonding between complementary bases - hairpin loop must be followed by a run of 6-8 U residues that form week bonds with the complementary run of 6-8 A residues in the DNA template - promotes dissociation of the RNA from the DNA template
question
Rho-dependent termination (prokaryote transcription)
answer
- Rho factor is a protein that terminates transcription - binds to the newly formed RNA and moves toward the RNA polymerase that has paused at a termination site - Rho then displaces the RNA polymerase and the 3'-OH end of the RNA - Rho factor has ATPase activity that is RNA dependent and it requires a polyribonucleotide that is greater than 50 bases long
question
structure of RNA polymerase (prokaryote transcription)
answer
- multisubunit enzyme exists in two forms: a core enzyme and a holoenzyme - core enzyme has four subunits and is capable of polymerizing ribonucleotides but does not recognize promoter regions in the DNA - holoenzyme has an addition sigma subunit that allows the enzyme to recognize promoter sequences
question
Requirements for RNA synthesis (prokaryote transcription)
answer
- requires all four ribonucleotides, a divalent cation (Mg2+ or Mn2+) and a template - double stranded DNA is the preferred template but single stranded DNA can be used - RNA polymerase does not require a primer and cannot proofread and correct mistakes
question
Function of RNA polymerase subunits (prokaryote transcription)
answer
- a subunits are involved in binding to the consensus sequences - B subunit contains the active site that binds nucleotide triphosphate and forms the phosphodiester bond of the polyribonucleotide chain
question
Transcription in prokaryotes: initiation
answer
- sigma subunit of the holoenzyme recognizes consensus sequences in the promoter, binds to the DNA and helps unwind the DNA double helix so that one strand can serve as a template - initiating ribonucleotide triphosphate is usually a purine (A or G) - after first few phosphodiester bonds are formed, the sigma subunit dissociates from the holoenzyme and the core enzyme begins elongation
question
Transcription in prokaryotes: elongation
answer
- core enzyme moves along the template that extends the RNA chain and the region of local unwinding moves with it - as the enzyme leaves a region, the DNA duplex reforms and RNA is displaced as a growing polynucleotide chain - growth of the chain is always in the 5' to 3' direction
question
Transcription in prokaryotes: termination
answer
- DNA template contains stop signals for transcription
question
Rifampin
answer
- antibiotic that inhibits initiation of RNA synthesis
question
Actinomycin D
answer
- antibiotic that binds to DNA and inhibits RNA synthesis by blocking movement of RNA polymerase along the template
question
RNA polymerase I (eukaryotic transcription)
answer
- localized in the nucleolus and transcribes the genes for three types of ribosomal RNA: 28S, 18S and 5.8S rRNA - rRNA is the most abundant RNA in the cell
question
RNA polymerase II (eukaryotic transcription)
answer
- found in the nucleoplasm, where it transcribes genes coding for proteins - primary transcripts synthesized by RNA polymerase II are known as heterogenous nuclear RNA, which are precursors for mRNA and snRNA - snRNA are part of the "spliceosome" a complex involved in removing intron sequences from precursor RNA molecules
question
RNA polymerase III (eukaryotic transcription)
answer
- located in the nucleoplasm and transcribes the genes for tRNA and 5s rRNA
question
Genes coding for protein
answer
- RNA polymerase II is found in the nucleoplasm, where it transcribes the genes whose RNAs will be translated into proteins - RNA polymerase II cannot initiate transcription by itself and is absolutely dependent on additional proteins, known as transcriptional factors, that act at promoter sites
question
RNA polymerase II initiation requires...
answer
- specific transcription factors bound to enhancer sequences - general transcription factors bound to promoter sequences - association of RNA polymerase II with transcription factors to form initiation complex
question
start site
answer
- initial nucleotide in position +1 of RNA transcript is usually A, flanked by pyrimidines
question
Promoters
answer
- two common sequences in promoters that are utilized by RNA polymerase II - TATA box- sequence of base pairs, which is important in the initiation of transcription is found in all eukaryotes. TFIID (critical transcription factor for RNA polymerase II) binds here - CAAT box: binding of transcription factors at this site may influence the formation of initiation complexes at other sites
question
Regulatory regions
answer
- sequences that increase or decrease the rate at which transcription is initiated by RNA polymerase II - usually located upstream from the start site but can also be internal to the gene or downstream from the gene - enhancer sequences bind transcription factors that increase the rate of transcription and silencer sequences bind factors that decrease the rate of transcription
question
Exons and introns
answer
- Exon: segment of the gene that is maintained in the mature mRNA and codes for protein - intron: portion of the primary RNA transcript that are removed by splicing together the exons
question
RNA polymerase III initiation requires...
answer
- transcription factors bound at internal promoter sequence and at start site - association of RNA polymerase III with transcription factors at the start site to form initiation complex
question
5' capping
answer
- 5' end of the RNA is "capped" shortly after the initiation of RNA synthesis - involves the addition of "inserted" methylated guanosine molecule to the first nucleotide in the RNA transcript - 5' cap plays an important role in the initiation of protein synthesis and in protecting the mRNA chain from degradation
question
Poly A tail of the 3'-OH end
answer
- mature mRNA molecules have a poly-A tail that is between 20 and 250 nucleotides long - tail is added to nRNA by the enzyme poly-A polymerase - increases the stability of nRNA - not all mRNA are polyadenylated (histone mRNAs are not)
question
RNA splicing
answer
- hnRNA contains coding sequences (exons) that are separated from one another by intervening sequences (introns) - in the conversion of hnRNA to mRNA. the introns are removed and the eons are spliced together - 5' cap and poly A tail are not removed during excision of introns - base sequence at the beginning of an intron is GU and at the end is AG - intron is excised as a loop or RNA that is degraded - after excision, ligation of the exons occur - reactions occur in the nucleus
question
operon in prokaryotes
answer
- set of structural genes and a regulatory region constitute an operon - structural genes code for a group of proteins required for a particular metabolic function - regulatory region is upstream (to the 5' side)
question
Lac operon
answer
- regulatory gene (i) codes for a repressor that can interact with operator sequence (O) - operator sequence is situated adjacent to the three structural genes (Z, Y and A) that code for three enzymes contender with lactose metabolism - expression of these three genes is regulated by the operator sequence
question
Negative regulation of the lac operon
answer
- in the absence of lactose, the repressor protein binds to the operator sequence and blocks the movement of RNA polymerase along the template, thus inhibiting transcription - when lactose is present, it induces transcription by the following mechanism. Lactose is converted to 1,6-allolactose which changes its conformation so it no longer binds to the operator region. Repressor is released and three structural genes are transcribed as a single mRNA that codes for all three proteins
question
Positive regulation of the lac operon
answer
- function of B-galactosidase in lactose metabolism is to cleave lactose to glucose and galactose and galactose is ultimately converted to glucose - if the bacteria has both glucose and lactose, there is no reason to activate the lac operon - when glucose is low, the lac operon is activated and the bacteria begins to use lactose to generate glucose. Effects of glucose are mediated by cAMP - when glucose is low, cAMP is high and when glucose is high, cAMP is low - when cAMP is high, it binds to a protein CAP. CAP-cAMP complex activates transcription of the lac operon by binding to the promoter region and allowing RNA polymerase to initiate transcription - CAP-cAMP complex is a positive regulator of the lac operon



