Cancer Set – Flashcards
Unlock all answers in this set
Unlock answersquestion
what are the 6 characteristics associated with anaplasia?
answer
pleomorphism and giant cells high nuclear/cytoplasmic ratio chromatin is clumped and prominent nucleoli are prominent abnormal mitotic figures distorted architecture
question
what is the Warburg hypothesis
answer
even in the presence of O2 cancer cells undergo glycolysis
question
what gene(s) is associated with non-polyposis coli ? what does this mutation end up causing?
answer
mutation in the DNA mismatch repair genes including hMSH1, hMLH1 and PMS2. these mutations cause microsatellite instability ( mutations of the short tandem repeat sequences distributed within the genome)
question
what do germline mutations in the APC gene predispose individuals to? what about somatic mutations? what is the role of APC ?
answer
mutations that are germline predispose to adenomatous polyposis coli somatic--> sporadic colon cancer APC protein serves as a platform for GSK-3beta. this allows catenin to stick around and be phosphorylated and subsequently cleaved by GSK-3Beta. CATENIN MEANS CHAIN: if not destroyed it chains APC and prevents its migration, however when destroyed, APC stimulates cell migration to the top of the villi. in colon cancer B catenin does not get degraded because APC is miss-shaped and cannot recruit GSK-Beta. Catenin prevents migration and also migrates to the nucleus where it promotes proliferation. this mechanism is very similar to that of an embryonic cell, however , in normal embryonic cells you have signasl such as the Wnt-Wnt signals which inactivate the GSK-Beta. in cancer there is no signal.
question
what kind of gene is Retinblastoma gene?
answer
tumor suppressor when Rb is hypophosphorylated it inactivates E2F (proliferation factor). If Rb is phosphorylated on the other hand--> frees E2F --> proliferation--> retinoblastoma NB: TGF-beta can inhibit the proteins that phosphorylate Rb--> prevent proliferation
question
p53 what does it normally do? what is the concept of dominant negative action? when is a mutation in p53 recessive?
answer
normally p53 forms homodimers that induce apoptosis. however when p53 is mutated (by UV light for example) it will either not be able to form a dimer ( if the top part is mutated) or it can form a growth promoting heterodimer when the DNA binding site is mutated in one of the p53 molecules : this is referred to as the dominant negative action of p53
question
VHL
answer
tumor suppressor gene that when mutated cause von Hippel Lindau disease--> tumor of malformed blood vessels in the brain or vascular tumors in the kidneys. normally protein VHL inhibits the Hypoxia Inducible Factor which is responsible for angiogenesis in places that are hypoxic ( makes sense, you want to bring oxygen to places that need it) . when HIF is active --> activates genes for EPO, VEGF, cMET and CXCR4. VEGF is the main factor
question
patient is diagnosed with an hemangioma. upon history taking you find out his father died of a brain hemangioma and so did his grandmother. what mutation is he most likely to have?
answer
VHL
question
what gene is deleted in 50% of pancreatic cancers?
answer
DPC4 (Tgfbeta gene)
question
the TGF-beta pathway is mutated in 100% of which cancers?
answer
colon cancers
question
what kind of cancer can develop from NF1 tumor suppressor mutation?
answer
sarcomas
question
what tumor suppressor genes are involved in cancer?
answer
APC, p53 , Rb, VHL, DPC4, bax, GAP , PTEN
question
what is the main gene involved in bladder cancer? is it a tumor suppressor gene or oncogene?
answer
RAS-oncogene RAS becomes an oncogene when it is locked into an active state and does not allow hydrolysis of GTP after tyrosine kinase receptor activation by growth factors.
question
what are three ways by which a protooncogene can become an oncogene?
answer
point mutation (RAS has an amino acid change)--> often due to environmental mutagens such as UV light amplification-->more copies of the gene= more product genetic rearrangements (usually translocation of chromosomes) simetimes viruses can insert their promoters in front of protooncogenes, and increase significantly their expression making them oncogenes
question
give one example of chromosomal translocation which leads to cancer
answer
C-myc is usually on chromosome 8. if a piece of chromosome 8 and a piece of chromosome 14 break off--> there is a translocation and now C-myc is in front of the immunoglobin gene--> Burkitts lymphoma philadelphia chromosome: CML leukemia: chromosome 9 and 22 C-Myc(8)/IgH(14)--> burkitt's Bcr (22) / Abl (9) --> CML
question
what is the cause of Burkitts lymphoma
answer
translocation of chromosome 8 containg C-myc with Chromosome 14 where immunoglobulins are encoded.
question
T/F chemical carcinogens often cause damage in their original form.
answer
F. it is the metabolism by our own enzymes that leads to the formation of the actual compound that damages the DNA
question
HOw does our body detoxify carcinogens?
answer
oxidation reduction hydrolysis hydration of the original compound. --> this can cause reactive oxygen species that are in fact carcinogenic conjgation--> often in the liver, so it becomes water soluble excretion--> usually kidneys
question
not all people who smoke develop cancer. why?
answer
you need an initiation factor: a driving mutation. intiation (such as for example a mutation in the APC gene) MUST occur before various other carcinogenic promoters (passenger mutation) , otherwise these will have no effect.
question
how is Paget's disease different from other metastatic cancers?
answer
it spreads intraepithelially rather than lymphatically like most other tumors
question
A 35 year old woman has thickening of the endometrial lining. you want to know if it's hyperplasia or neoplasia, what do you look for?
answer
different isotypes of GD6P since it is X linked. there should be a 1:1 ratio. if the ratio is off--> neoplasia
question
what is differentiation therapy?
answer
when you aim to differentiate cancer cells so they stop dividing.
question
what's a gain of function mutation?
answer
when a mutation in a proto-oncogene imparts a whole new function to the resulting protein that will be oncogenic
question
what protein that regulates apoptosis is mutated in familial gastric cancer?
answer
E-Cadherin
question
Since the age of 5 a young girl continues to develop numerous cancers one after the other. what is she affected with? if the doctor was to sequence her genome where would he find an abnormality?
answer
Li-Fraumeni syndrome p53 is mutated, no apoptosis
question
ii. What are some DNA repair genes that can be mutated to cause cancer?
answer
1. BRCA1 and BRCA 2 mutations can predispose families to familial breast and ovarian cancer. 2. DNA mismatch repair at the germline level can cause hereditary nonpolyposis colon carcinoma. 3. WT1 can be mutated to cause familial Wilms tumor. 4. MEN1 is another DNA repair gene that can be mutated to cause cancer. 5. hMSH1, hMLH1 and PMS2. these mutations cause microsatellite instability ( mutations of the short tandem repeat sequences distributed within the genome)--> non polyposis coli
question
what are mutations that contribute to the development of malignant phenotype called?
answer
driver mutations
question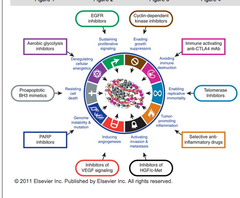
what are the hallmarks of cancer?

answer
i. Cancer cells avoid immune destruction due to various mutations. ii. They evade growth suppressors. iii. Cancer cells enable replication to occur indefinitely leading to immortality. iv. These cells have tumor promoting inflammation. v. Cancer cells have activating invasion and metastasis. vi. Cancer cells also exhibit genomic instability. vii. Cancer cells induce angiogenesis because they need oxygen and nutrients. viii. Malignant cells resist cell death. ix. There's deregulation of cellular energetics in cancer cells as well. x. There's sustained proliferative signaling in cancer cells too.
question
X is to RAS as Y is to PI3K
answer
X=Gap Y=Pten PTEN inhibits protooncogene PI3K, while GAP regulates RAS
question
54 year old man comes into your office complaining of tiredness and slight abdominal pain. you run a CBC electrolyte panel and see that he has microcytic anemia and iron deficiency. upon questioning he tells you that he has not lost any blood recently. what is your next step?
answer
colonoscopy. right side colon cancer can manifest with anemia, in later stages as microcytic anemia.
question
patient is brought to the ER in excruciating pain from his abdomen. He tells you that he has been a bit constipated lately. and that he cannot keep anything down, and keeps vomiting. his CBC shows microcytic anemia. what do you suspect?
answer
left side colon cancer
question
patient comes into your office complaining of morning diarrhea. he tells you that every morning in the past month he wakes up and needs to evacuate pronto. he also tells you that whenever he goes to the bathroom he feels like he has not pooped everything out, as if there is something always there. what do you suspect and what test would you run? what is the chance that when you run a CBC this patient is anemic?
answer
do a rectal exam you suspect colorectal cancer high chance of anemia since this type of cancer shows bleeding in 88% of cases
question
HIV positive patient comes to your office worried because he saw blood on his toilet paper after wiping. you do a CBC and he is not anemic. what do you worry about ?
answer
anal cancer squamous cell carcinoma (not adenocarcinoma)
question
60 y.o. male patient comes to your office complaining of difficulty urinating and having to pee several times at night. you run a blood test and PSA levels are slightly elevated. what exam do you perform?
answer
rectal, check for prostate cancer.
question
patient comes to the ER complaining of blood in the urine, flank pain and abdominal mass.the blood in the urine is more prevalent in the morning. on PE you have +CVA tenderness. Patient HX is positive for father who died of brain hemangioma. what do you suspect?
answer
VHL Von Hippel Lindau syndrome and renal cancer
question
patient is a smoker who presents to your office for a physical. in the urine analysis you see signs of microscopic hematuria. what do you suspect?
answer
bladder cancer
question
19 y.o. kid comes to your office saying that the other day he felt a mass in his scrotum. you tell him that he was really smart to come immediately. why? what do you suspect?
answer
suspect testicular cancer. smart that he came soon because it can metastasize to the brain if neglected.
question
46 year old patient, who is a breast cancer survivor, comes to your office complaining of irregular bleeding outside of her menstrual period. she thinks she might be pregnant. you run a pregnancy test, but it's negative. what additional tests would you like to perform?
answer
this patient has signs of uterine cancer that may have been caused by tamoxifen (hormone blocker often given to women with breast cancer). you want to do an endometrial biopsy.
question
patient comes to the ER, his right eyelid is drooping, pupils are unequal, with the right pupil being 2mm and non reactive. He tells you his right pinky has been hurting. What do you suspect?
answer
Pancoast tumor with horner syndrome --> usually squamous cell carcinoma
question
patient comes into the ER. his face is flushed, his neck veins are engorged, and his arms are swollen bilaterally. you call the attending because this is a medical emergency. what do you suspect?
answer
well it could be cardiac tamponade but considering we are in the cancer unit, I am going to say SVC syndrome NB: you might want to use aggressive chemo to treat this if it is SCLC.
question
patient comes to the ER. he is confused, and has peripheral edema. you suspect renal failure and when you run a blood test you think he is in DIC. what do you fear?
answer
Thrombocytopenic purpura due to paraneoplastic syndrome associated with lung cancer
question
what are two cancers that can cause SVC syndrome?
answer
lung and esophageal.
question
what cancers are associated with acanthosis nigricans?
answer
lung and gastric cancer
question
patient comes to your office complaining that in the past month he starts itching every time he drink beers. you ask him if he is also experiencing night sweats, why?
answer
Hodgkin Lymphoma
question
which cancer is most likely associated with fever?
answer
renal cell cancer
question
What drug can cause renal failure? what about cardiac failure?
answer
methotrexate and doxorubicin
question
cyclophosphamide
answer
Alkylating agents Nitrogen mustards o EX: cyclophosphamide (most commonly used drug in class) o Administered in inactive form o Mechanism of action Activation via hydroxylation by hepatic CYP 450 enzymes this hydroxylation forms 4-hydrocyclophosphamide that can have 2 fates: 1. in most of non tumor cells, high levels of gutathion transferase and aldehyde dehydrogenase turn 4-OH-cyclophosphamide into an inactive compound OR 2. via non enzymatic reaction 4-hydrocyclophosphamide is turned into aldophosphamide--> phosphoamide mustard that can form a carbonium and be attacked by electrons from N7 of guanine. the formation of the toxic compound is prevalent in cancer cells because they produce way less metabolic enzymes which would inactivate 4-hydrocyclophosphamide RESULT: apoptosis
question
carmustine (BCNU)
answer
Contain urea and nitrogen o Administered in inactive form o Mechanism of action Spontaneous activation(no need for liver enzymes)-->2 degradation products One alkylates guanine in DNA Another carbamoylates cell proteins ( covalently modifies them) RESULT: apoptosis
question
cisplatin
answer
platinum coordination complex o Higher activity at S phase of cell cycle- although it affects all phases because it prevents DNA transcription. o Central platinum molecule with 2 chloride groups (-Cl) + 2 amine groups (NH2) via acquation reaction--> Cl is replaced by hydroxyl groups Each -OH reacts with DNA intrastrand, interstrand crosslinks Guanine-guanine Guanine-adenine mostly interferes with S phase however can also interfere with transcription: Inhibition of RNA synthesis, transcription cell apoptosis
question
methotrexate
answer
MOA: folic acid analog, competitively inhibits dihydrofolate. its polyglutamated forms ( which build up inside the cells making this drug more toxic) inhibits also thymidine synthase, GAR transfromilaste and AICAR transformylase 1.thymidine synthase inhibition prevents the formation of TMP. thymidine synthase is ihhibited directly by the polyglutamated forms of methotrexate and by the buildup of dihydrofolate polyglutamate (FH2Glun) and TMP. GAR transformylase inhibition prevents the formation of AICAR from PRPP and aspartate and AICAR transformylase inhibition prevents the formation of inosine monophospate which is a purine precursor
question
leucovorin
answer
rescues host cells from toxicity by methotrexate it is a tetrahydrofolate that doesnt need dihydrofolate reductase. therefore it rescues the host cell from apoptosis and allows it to generate TMP and IMP. important to note that also cancer cells benefit from leucovorin, however due to their higher metabolism, they will be less affected by leucovorin.
question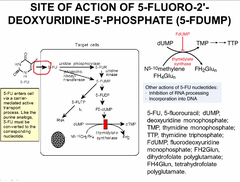
5-FLUOROURACIL

answer
Some bases (uracil, guanine) can be synthesized de novo then converted to nucleotides and nucleosides 5 fluorouracil is a BASE ANALOGUE like 6-Mercaptopurine o Ex) 5-FLUOROURACIL can be provided as a base analog that will be converted into a nucleoside then nucleotide analog that can be incorporated into the DNA 1. 5-flurouracil can act on the pathway of RNA synthesis. a. 5-flurouracil when it enters the cell is converted into floxuridine. b. Floxuridine is phosphorylated to floxuridine monophosphate (FUMP). c. FUMP is phosphorylated to floxuridine diphosphate (FUDP). d. FUDP is phosphorylated to floxuridine triphosphate. e. FUTP becomes incorporated into the RNA chain. Because of the fluoride atom that FUTP contains, the RNA will have modified properties and will not be able to be translated into protein. 2. FUDP can go down another pathway to be transformed by ribonucleotide reductase so that it goes down the path of DNA synthesis. Ribonucleotide reductase removes a hydroxyl group on FUDP to give you fluorodeoxyuridine diphosphate (FdUDP). a. FdUDP can be incorporated into the DNA (after being phosphorylated to FdUTP) which will modify the properties of DNA because of the presence of the fluoride atom. b. However FdUDP can also be dephosphorylated to generate fluorodeoxyuridine monophosphate (FdUMP). FdUMP is actually an inhibitor of thymidylate synthase. FdUMP can be generated by starting with 5-flurouracil as well. But in any case FdUMP will inhibit the same enzyme that's targeted by the folic acid analogs. So FdUMP blocks the generation of TMP which is an essential building block of DNA.
question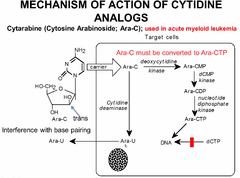
cytarabine- C-ara

answer
cytosine analog this is a nucleoside analogue. unlike uracyl and guanine (which can be given as single bases and turned into nucleoside-->tide) cytidine, adenine and thymine cannot. C-Ara is a nucleoside analogue that is turned into a nucleotide by the cell. in order to enter the cell and exert its effect: 1. carried into the cell by specific carriers 2. has to be turned into Ara-CTP (phosphorylated 3 times for it to be incorporated into DNA, where it competitively inhibits dCTP from being incorporated. because of the trans position of C2, it cannot rotate on the helix and allow proper binding to Guanine. NB: because some of the Ara-C is converted into Ara-U and excreted from the cell instead of being phosphorylated,--> YOU NEED TO GIVE HIGH amounts of the DRUG to patients
question
6-Mercaptopurine
answer
6-Mercaptopurine (Purine Analog) - Mechanism of Action Essential modification is -SH group In order to be toxic 6-MP must be converted into nucleoside and then nucleotide HOWEVER, T(IMP) is a poor substrate for guaisyl kinase, therefore instead of being phosphorylated further, it accumulates and becomes toxic: 6-MP can also lead to decreased synthesis of AMP and GMP o 6-MP structure is sufficiently similar to AMP and GMPinduces feedback inhibition inhibition of further production of AMP and GMP
question
which nucleotide analogue can be given as bases only? which ones require to be nucleoside or nucleotide?
answer
bases only: uracyl and guanine: mercaptorpurine is given as a base and so is fluorouracyl
question
taxol
answer
TAXOL is different from other drugs (in above table) o Binds to microtubules and stabilizes themdoesn't allow them to show instability or treadmillingBLOCKS MITOSIS
question
vinblastin, vincristine
answer
Bind free tubulin dimersdon't allow them to polymerize into microtubulesentire microtubule will shrinkMITOSIS WILL NOT PROCEED
question
Antitubulins - Resistance mechanisms
answer
Antitubulins - Resistance MUTATIONS IN BETA-TUBULIN o Tubulin no longer binds to drugs INCREASED EXPRESSION OF MDR-1 GENE (encodes the P-glycoprotein) o P-glycoprotein is an efflux pump that pumps out drugs o Increased P-glycoprotein results in multi-drug resistance Not just resistant to antitubulins but many other drugs as well
question
dactinomycin
answer
Dactinomycin (also known as Actinomycin D) - first antibiotic used in anticancer therapy - structure o polypeptide, contain 3 cyclic polypeptide ring o chromopeptide - has a yellow-red color - mechanism of action o binds to DNA double helix and forms dactinomycin-DNA complex o blocks transcription and replication of DNA by DNA and RNA polymerases o causes single-stranded breaks in DNA through two mechanisms: generates free-radical intermediates that oxidize and break DNA binds to topoisomerase II, blocking its function o acts on rapidly proliferating cells - may produce alopecia - when extravasated subcutaneously, can causes marked local inflammation - does not cross blood-brain barrier CANNOT BE GIVEN TO TREAT BRAIN TUMOR
question
Doxorubicin
answer
MOA: forms a tripartate complex with DNA and topoisomerase II --> cleaves DNA--> apoptosis analogs include daunorubicin, idarubucin, epirubicin ALL OF them have quinone and hydroquinone moieties on adjacent rings which permit the loss or gain of electrons: makes them very reactive and toxic to the HEART!! doxorubicin, by virtue of its quinone groups, esp in the presence of Fe--> create free radicals--> attack DNA and oxidize it
question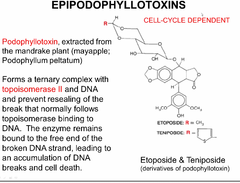
epipodophyllotoxins (ETOPOSIDE and TENIPOSIDE)

answer
same mechanism of doxorubicin by forming a tripartate with topoisomerase II and DNA
question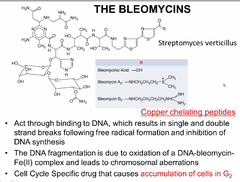
Bleomycins

answer
also likes like doxorubicin with the quinone groups BLOMYCINS need copper or iron in order to exert oxidizing activity. therefore in someone with copper deficiency this may exacerbate symptoms of leukopenia, neutropenia and anemia or just simply not work. Preferentially they work in G2
question
imatinib
answer
In CML there is constitutive action of Abelson kinase on chromosome 9. There is also a break point cluster region gene on chromosome 22. The fusion of chromosome 9 and 22 forms the Philadelphia chromosome, and leads to the production of a chimeric protein that keeps Abelson kinase active. Because of this mutation, cells keep proliferating. One drug therapy developed is called imatinib. This drug targets proteins and blocks the activity of Abelson kinase. This blocks proliferation of the tumor cell. Unfortunately, resistance to this drug has developed. In the bone marrow, when a CML cell appears, clonal expansion leads to increase numbers. Imatinib reduces proliferation. But additional mutation may allow tumor cells to activate another proliferative pathway that does not rely on Abelson kinase. This mechanism of resistance means now the drug does not work and the tumor can re-grow.
question
what two cancer are treated with tyrosine kinase inhibitors?
answer
EGFR --> lung cancer CML--> imatinib
question
what are 2 enabling characteristics of cancer that enable the other 8 hallmark of cancers to be achieved?
answer
tumor promoting inflammation--> recall that VEGF is released during chronic inflammatory processes and cancers take advantage of that for angiogenesis genome instability and mutaiton
question
what are the therapeutic agents that target these specific hallmarks of cancer: 1.Sustaining proliferative signaling 2.Enabling replicative immortality 3.tumor promoting inflammation 4.inducing angiogenesis
answer
1. TKI (EGFR example) 2. inhibit telomerase activity 3. antiinflammatory drugs 4. inhibit VEGF
question
what are the 2 main features that allow anti-cancer drugs to target cancer cells over benign cells of the body?
answer
1. selectivity for high division rate of cancer 2. increased metabolism in cancer cells
question
what order of kinetics does cell death in cancer cell follow?
answer
VERY important: 1st order kinetics. why important? That means that if you give a drug to a bunch of cancer cells, you kill half of them, and then kill half of the remaining population, and so on. This is what first order kinetics means. In the end if you just have 2 cells left and give the anti-cancer drug, you will kill one of the two cells. If just one cancer cell is remaining after treatment, it is fully capable of regenerating the tumor. YOU NEED TO KILL ALL OF THEM FOR THE CANCER TO NOT COME BACK!!
question
what do most of the alkylating agents contain? (functional group). are alkylating agents active as they are?
answer
functional group is bis-(2-chloroethyl)group not active, need to be activated and form a reactive carbonium ion. all of the mustard agents are inactive, need to be activated in the liver by cytochrome p450 that attaches hydroxyl group on drug via hydroxilation
question
patient is diagnosed with Anaplastic oligodedroglioma. his MRI shows enhancement of the lesion. would you give high or low doses of methotrexate to cure this type of cancer?
answer
YOU DONT GIVE METHOTREXATE AT ALL: You only reach 3% of the blood concentration of methotrexate in the CSF. So methotrexate is not an effective drug for tumors of the CNS. treat with chemotherapy that was not specified in lecture 32.
question
what are some resistance mechanisms to methotrexate?
answer
1.impaired transport of methotrexate into cells 2.production of altered forms of DHFR that have decreased 3.affinity for the inhibitor 4.increased conc of intracellular DHFR enzyme 5.decreased ability to synthesize methotrexate 6.polyglutamates 7.increased efflux.
question
thymidylate synthase is a target for: a)methotrexate b)6-mercaptopurine c)5-fluoracil d) a & C e) all the above
answer
d. In 5-flurouracil, you replace the methyl group of thymine with a fluoride atom. 5-flurouracil blocks thymidylate synthase. Thymidylate synthase is thus a target of both 5-flurouracil and methotrexate.
question
what are 2 anticancer drugs that do not cross the BBB, or that have limited therapeutic benefits in the tx of brain tumors?
answer
dactinomycin and methotrexate
question
driver mutations
answer
mutations that contribute to the development of malignant phenotype
question
RAS
answer
oncoproteins ( growth factor receptor--> when activated by mutations --> cancer inhibited by GAP
question
GAP
answer
inhibits ras
question
PI3K
answer
oncoproteins ( growth factor receptor--> when activated by mutations --> cancer
question
PTEN
answer
inhibits PI3K
question
MYC
answer
oncoproteins ( growth factor receptor--> when activated by mutations --> cancer
question
D cyclins
answer
oncoproteins ( growth factor receptor--> when activated by mutations --> cancer
question
where, what, how and why: synovial sarcoma
answer
where: chromosome 18 and X what: translocaton t(18;X)(p1.2;q11.2) creating a fusion transcript how: karyotype FISH RT-PCR Why: diagnostic, follow up and potential targets for new therapy
question
well to moderately differentiated lung adenocarcinoma
answer
where: EGFR in chromosome 7p12 What: deletions in Ex 19 and point mutation in Ex21 How: capillary electrophoresis fragment analysis or pyrosequencing why: responds to targeted therapy
question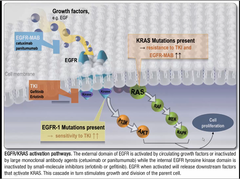
waht two types of genetic abnormalities in lung cancer have therapeutic potential? how does treatment change according to what protein is involved?

answer
EGFR --> give tyrosine kinase inhibitor KRAS--> DO NOT GIVE TKI, because it has resistance to IT
question
EGFR
answer
transmembrane glycoprotein encoded in Chromosome 7 it's an oncogene--> perpetual activation leads to cancer this protein is ubiquitus involved in cellular growth, cell cycle control and apoptosis transmembrane, with a tyrosine kinase intracellular domain MUTATIONS ( 7p12) causes lung adenocarcinoma--> cna be treated with tyrosine kinases
question
what do you use to see EGFR exon 19 deletions?
answer
microsatellites study
question
anaplastic oligodentroglioma WHO grade III

answer
where: deletion chromosome 1p and 19q what: deletions How: loss of heterozygosity FISH Why: predicts response to therapy
question
how do you measure loss of heterozygosity?
answer
compare chrmomosomes tumor cells with blood you should have an allele from mom and one from dad. in a tumor that arises from a chromosomal deletion the affected cells only have one allele
question
patient has a colorectal tumor. EGFR is mutated, how do we treat? what if EGFR was not mutated? would have our tx plan have changed?
answer
EGFR mutated: use tyrosine kinase inhibitor EGFR not mutated: treat more aggressively
question
what is small cell lung carcinoma mostly associated with?
answer
smoking (represents 20% pf all lung cancers)
question
80% of lung cancers are Non-small-cell-lung-carcinoma, what are the three major subtypes ? which one can be targeted with tyrosine kinase inhibitors?
answer
1 adenocarcinoma--> if EGFR positive-->TKI 2. large cell 3.Squamous cell
question
can you use tyrosine kinase antibodies if the lung adenocarcinoma is due to KRAS?
answer
no, that would be bad. this type of cancer is very resistant to TKI--> potentiate the EGFR pathway
question
what is upregulated in an EGFR adenocarcinoma? where would we look for mutations in a EGFR carcinoma?
answer
PI3K and ACT mutations: deletion of 19 (50%) point mutations in 21 or 18 duplication and insertions in 20
question
patient comes to the ER complaining of headaches and generalized seizures for the past month. you do a CT scan with contrast and you notice an enhancing mass. You promptly call the onco team. what is abnormal about the scan apart from the mass?
answer
enhancement of the mass. this means that the BBB has been altered/breached. Angiogenesis secondary to the tumor will allow contrast to enter the tumor.



