Biochemistry: Water Properties – Flashcards
Unlock all answers in this set
Unlock answersquestion
three physical states (phases)
answer
liquid, solid, and gas
question
freezing & melting point of water
answer
32° Fahrenheit, 0° Celsius
question
boiling point of water
answer
212°Fahrenheit, 100° Celsius
question
polar

answer
molecule where electrons ARE held more by one atom than another. Electrons are not shared equally (not balanced). Water and sugar are examples.
question
nonpolar

answer
molecule where electrons ARE NOT held more/less strongly by different atoms. Electrons are shared EQUALLY (balanced). Sulfur and oil are examples
question
solvent

answer
chemical that can dissolve another chemical into itself
question
universal solvent
answer
many chemicals, both ionic and polar, can dissolve in water
question
cohesion

answer
water molecules "stick" together
question
adhesion
answer
water also "sticks" to other substances
question
surface tension
answer
force of the water layer- you can lay a pin on the water's surface
question
capillary action

answer
able to move up through a tube against gravity
question
density
answer
ratio of mass/volume; how close together or spread apart atoms or molecules are. Liquid water is more dense than solid water (ice) and gas water (vapor)
question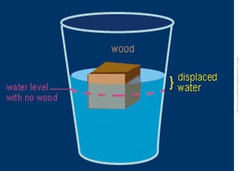
buoyancy

answer
upward force of an object in a fluid (floating/sinking). ice floats on water
question
specific heat (insulation)
answer
Energy needed to heat things. Water holds heat very well. it takes a long time to heat up (use energy) and a long time to cool down (release energy)
question
temperature (thermal) homeostasis
answer
able to control temperature with water- keeps you cool when you are heating up (sweating), keeps the temperature in your cells balanced
question
Earth- water composition
answer
70%, but 97% of water is salt water
question
human body- water composition
answer
60-70%



