Chemistry – Nuclear Chemistry Test – Flashcards
Unlock all answers in this set
Unlock answersquestion
Which radioactive nuclide is used to treat cancer?
answer
cobalt-60
question
How many half-lives are required for three-fourths of the nuclei of one isotope in a sample to decay?
answer
2
question
Gamma rays are
answer
electromagnetic waves.
question
Beta particles are
answer
electrons.
question
The half-life of an isotope is the time required for half the nuclei in a sample to
answer
undergo radioactive decay.
question
In a nuclear reaction, unstable nuclei change their number of protons and neutrons,
answer
give off large amounts of energy, and increase their stability.
question
In nuclear chemistry, an atom is referred to as a
answer
nuclide.
question
In nuclear reactors, the role of control rods is to
answer
absorb neutrons or slow down the reaction.
question
Which statement does not describe fission?
answer
Stable, lightweight nuclei start the process.
question
What is the function of shielding in a nuclear reactor?
answer
to contain radiation
question
Alpha particles are
answer
helium nuclei.
question
What is the half-life of an isotope if 125 g of a 500 g sample of the isotope remain after 3.0 years?
answer
1.5 years
question
A nuclide is identified by
answer
the number of protons and neutrons in its nucleus.
question
Among atoms with low atomic numbers, what is the neutron-proton ratio of the most stable nuclei?
answer
1:1
question
Radioactive tracers in fertilizers can be used to measure
answer
how well the fertilizer is absorbed by plants.
question
In an artificial transmutation, what is required to bombard nuclei with positively charged alpha particles, protons, and other ions?
answer
great quantities of energy
question
What unit measures radiation damage to human tissue?
answer
rad
question
The energy released in a nuclear reaction comes from
answer
the binding energy of the nucleus.
question
All radioactive nuclides undergo
answer
radioactive decay.
question
Which of the following generally have the lowest penetrating ability?
answer
alpha particles
question
What does the 218 in polonium-218 represent?
answer
the mass number
question
Which of the following has the greatest penetrating ability?
answer
gamma rays
question
OA(FL) = AL
answer
OA = original amount, FL = fraction left, AL = amount left
question
N = log(FL) / log (.5)
answer
N = number of half-lives, FL = fraction left
question
(1/2) ^ N = FL
answer
N = number of half-lives, FL = fraction left
question
N = Time gone by / Time of 1 half-life
answer
N = number of half-lives
question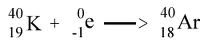
K-capture
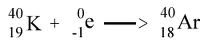
answer
EX:
question
How do nuclear power plants actually produce power/electricity?
answer
Water is pulled in from a river/water source and turned into steam, which then drives turbine generators to produce electricity. The difference is the source of heat. At nuclear power plants, the heat to make the steam is created when uranium atoms split - called fission. Also, the nuclear aspect never comes in contact with the water/steam.
question
Mass Defect
answer
Difference between the mass of an atom and the sum of the masses of its particles
question
Nucleon
answer
Protons and neutrons
question
Roentgen (R)
answer
Unit used for measuring radiation exposure



