plumbic perchlorate hydrate H2ClO5Pb structure – Flashcards
Flashcard maker : Judith Simpson
Contents
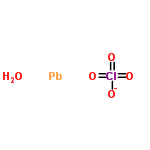
| Molecular Formula | H2ClO5Pb |
| Average mass | 324.664 Da |
| Density | |
| Boiling Point | |
| Flash Point | |
| Molar Refractivity | |
| Polarizability | |
| Surface Tension | |
| Molar Volume |
- Experimental data
- Predicted – ACD/Labs
- Predicted – ChemAxon
- Miscellaneous
Predicted data is generated using the ACD/Labs Percepta Platform – PhysChem Module
No predicted properties have been calculated for this compound.
| Density: | |
| Boiling Point: | |
| Vapour Pressure: | |
| Enthalpy of Vaporization: | |
| Flash Point: | |
| Index of Refraction: | |
| Molar Refractivity: | |
| #H bond acceptors: | |
| #H bond donors: | |
| #Freely Rotating Bonds: | |
| #Rule of 5 Violations: |
| ACD/LogP: | |
| ACD/LogD (pH 5.5): | |
| ACD/BCF (pH 5.5): | |
| ACD/KOC (pH 5.5): | |
| ACD/LogD (pH 7.4): | |
| ACD/BCF (pH 7.4): | |
| ACD/KOC (pH 7.4): | |
| Polar Surface Area: | |
| Polarizability: | |
| Surface Tension: | |
| Molar Volume: |
Click to predict properties on the Chemicalize site


