Physical Science -Unit 6- Atomic Structure & Periodic Table SPS1.a.-c. – Flashcards
Unlock all answers in this set
Unlock answersquestion
Atomic number

answer
the number of protons (+) in the nucleus of an atom of a given element and equal to the number of electrons (-) surrounding the nucleus.
question
Atomic Mass or Mass Number

answer
Sum of the number of protons and neutrons
question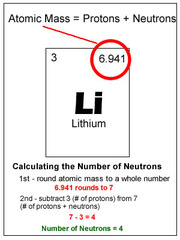
How to find the number of neutrons in an atom?

answer
Atomic mass number - Atomic number
question
Neutrons
answer
A neutrally charged subatomic particles in the nucleus of an atom
question
Protons
answer
A positively charged subatomic particle in the nucleus of an atom
question
Isotopes
answer
Atoms of the same element with different numbers of neutrons
question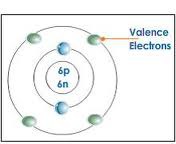
Valence electrons

answer
the number of electrons in the outermost energy level
question
Atomic structure
answer
Atoms are made of a nucleus of protons and neutrons orbited by electrons
question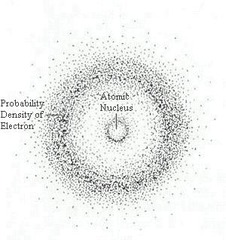
Electron cloud

answer
a region around the nucleus of an atom where electrons are likely to be found
question
The number of neutrons in a U-238 isoptope
answer
146
question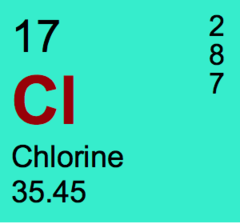
The number of electron in an atom of chlorine

answer
17
question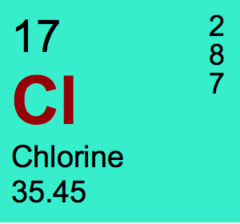
The number of protons in an atom of chlorine.

answer
17
question
The number of neutrons in an atom of chlorine
answer
18
question
Period
answer
A horizontal row of elements in the periodic table - determines how many electron energy levels an atom has
question
Group or Chemical Family
answer
A vertical column on the periodic table which share a common number of valence electrons and react the same
question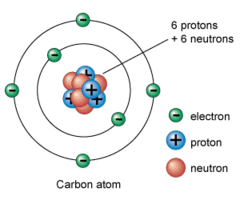
Atom

answer
Smallest particle of an element
question
Ion
answer
An atom or molecule with a net electric charge due to the loss or gain of one or more electrons.
question
Electrons
answer
Negatively charged particles found outside the nucleus
question
Metals
answer
Elements that have a shiny or metallic luster, are malleable, ductile, and are good conductors of heat and electricity
question
Nonmetals
answer
Elements, mostly gases, or dull and brittle solids that that are poor conductors of heat and electricity.
question
Metalloids
answer
Elements along the staircase. Have properties of both metals and nonmetals. Ex: Silicon
question
State the number of neutrons in each of these Lithium isotopes - Li-6, Li-7, & Li-8
answer
3, 4, & 5
question
Gold (Au) forms two common ions - how many electrons does each have: Au 1+ and Au 3+

answer
Au 1+ has 78 electrons, Au 3+ has 76 electrons



