PBDA Fall 2016 ISU college of Pharmacy
Unlock all answers in this set
Unlock answersquestion
Alcohol

answer
physical and chemical properties Bonding-alcohols will have a permanent dipole interaction which will result in hydrogen bonding to other molecules. The more hydrogen bonding that occurs, the higher the boiling point. Alcohols are relatively stable compounds, except in the presence of an oxidizing agent ( a substance that gets reduced by gaining electrons)
question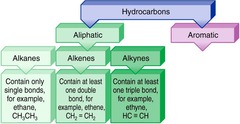
aliphatic hydrocarbons

answer
Description these are single linear hydrocarbon chains ranging from alkanes, alkenes, and alkynes. (sp1, sp2, and sp3 hybridization). Physical and Chemical Properties Bonding- The only inter molecular interaction that can exist for aliphatic hydrocarbons is Van der Waal bonding. Chemical They are considered stable in vitro conditions.
question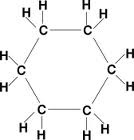
cyclic hydrocarbons

answer
hydrocarbons that form a cyclic ring, can be further classified as aromatic, non-aromatic, and anti-aromatic.
question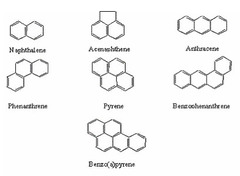
aromatic hydrocarbons

answer
will follow the 4n+2 rule (2,6,10,14....etc)
question
non aromatic hydrocarbons
answer
will not obey Huckle's rule. The cyclic hydrocarbon will not contain all sp2 hybridization.
question
anti-aromatic hydrocarbons
answer
will follow the 4n rule (4,8,12,16...etc)
question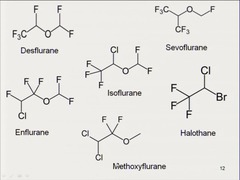
Halogenated hydrocarbons

answer
Hydrocarbon chain bonded with a halide(s). -Vaan der Waal inter-molecular bonding. -very lipophilic because hydrocarbons that are covalently bonded to a halide results increases the lipophilic properties of an organic structure. -they are stable but will react with a nucleophile (a substance that will donate a electron(s), or accept a proton. It is a Lewis base). -as the number of halogens increase on a hydrocarbon, flammability is decreased. (general aesthetics).



