MTBS – iGCSE Chemistry States of Matter/Diffusion/Separating Paper 1 & 2
Unlock all answers in this set
Unlock answersquestion
Describe the particles in a solid
answer
- Strong forces of attraction - Regular arrangement of particles - Fixed position - Vibrate (hotter the solid the more they vibrate) - Fixed volume and shape
question
Describe the particles in a liquid
answer
- weak forces of attraction between partciles - Randomly arranged - Free to move over each other - Fixed volume but can change shape - Higher temperature = paticles flow more freely
question
Describe the particles in a gas
answer
- particles are free to move apart - move in straight lines - Gases fill any container they are in - Higher temperature, faster moving particles = higher pressure
question
Changing state - describe melting (opposite of freezing)
answer
- Solid heated, particles vibrate more - Particles have enough energy to overcome the forces of attraction between partilces - Solid turns into a liquid
question
Changing state - describe boiling (oppoisite of condensing)
answer
- Liquid heated, particles move more - Particles have enough energy to overcome the forces of attraction between partilces - Liquid turns into a gas
question
What is the difference between boiling and evaporating
answer
- Boiling requires heating. - Evaporation happens at any temperature. (Puddles evaporate)
question
Sublimation
answer
Solid changes directly into a gas
question
What happens if a solid piece of potassium permangenate is placed into a beaker of water

answer
- Purple colour from the pottasium permangenate spreads ot over time - Potassium permangenate diffuses
question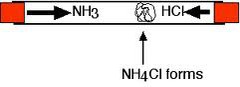
What happens when ammonia and Hydrogen chloride are put in a tube at opposite ends

answer
-Both gases spread out through the tube (diffusion) -Where they react a white solid Ammonium chloride forms - Ammonia (NH₃) diffuses faster than the Hydrogen chloride (HCl) because it is a smaller molecule. - The white solid appears nearer the Hydrogen Chloride end
question
What is the symbol equation for Hydrogen chloride reacting with ammonia
answer
NH₃(g) + HCl(g) ⇌NH₄Cl(s)
question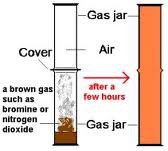
What happens when Bromine Liquid is put into a tube

answer
- The orange brown gas spreads out throughout the tube. - The bromine gas diffuses through the tube
question
What sort of reaction is Hydrogen chloride reacting with ammonia
answer
- A neutralisation reaction (It is also reversible)
question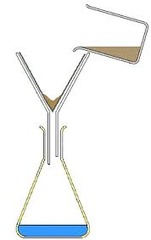
How could you separate a solid from a liquid

answer
Filtration Using filter paper to catch the solid
question
How could you separate a soluble salt from water
answer
crystallisation Heat the solution - increases the concentraiton of the solution Leave to slowly evaporate and crystallise Dry the crystals in air or a warm oven
question
What two ways could you use to separate a mixture of two liquids.
answer
- Chromatography - identifies the substances - Distillation - collects the liquids (fractional distillation for crude oil)
question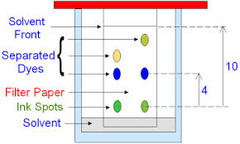
Describe how you would undertake chromatography

answer
Draw a pencil line near the bottom (pen would spread) Spot mixture onto line Place into solvent - solvent below the pencil line Cover the container Leave to separate
question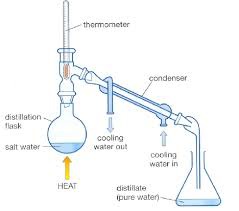
Describe how you would undertake distillation

answer
Heat the solution One of the substances evaporates It cools and condenses and collected
question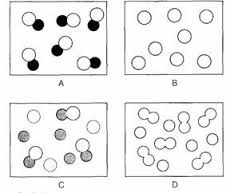
A - compound B - Element C - Mixture of compound and element D - Mixture of elements

answer
Element mixture or compound



