Lecture Exam 1 – INORGANIC CHEMISTRY – Flashcards
Unlock all answers in this set
Unlock answersquestion
major elements found in the body-96% of body mass
answer
oxygen, carbon, hydrogen and nitrogen
question
What is oxygen's % of total body mass
answer
65.0%
question
What is carbon's % of total body bass
answer
18.5%
question
What is hydrogen's % of total body mass
answer
9.5%
question
What is nitrogen's % of total body mass
answer
3.2%
question
What is the percentage of breakdown of water vs. inorganic substances vs organic substances
answer
water is 60% inorganic is 2% organic is 38%
question
What is the difference between elements and atoms
answer
each element consists of atoms
question
Ionic bond
answer
a chemical bond that forms as a result of the mutual attraction of oppositely charged atoms, forms when one atoms donates one or more outer-shell electrons to another atom.
question
covalent bond
answer
a bond in which sharing of atoms electrons between 2 atoms is unequal - can be polar or non polar.
question
non polar covalent bond
answer
Methane is an example - when 2 atoms of the same element share electrons equally.
question
polar covalent bond
answer
Water is an example - sharing of electrons between two atoms is unequal-atom with stronger attraction has partial negative charge - the atom with lesser attraction has partial positive charge.
question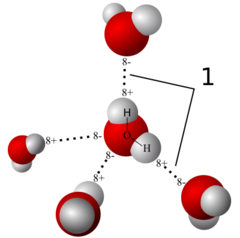
Hydrogen bond

answer
weak compared to ionic and covalent. forms polar covalent bonds usually between H, O, and N.
question
Important life properties of water
answer
high heat capacity, reactivity (solvent, hydrolysis, dehydration, high heat vaporization, polar solvent properties, cushioning
question
What is the chemical structure of water
answer
2 hydrogen atoms and one oxyen atom in a polar covalent bond. Postive charge.
question
exergonic chemical reaction
answer
a chemical reaction that releases more energy than absorbed and is often used to drive an endergonic reaction. (ATP is an example)
question
endergonic chemical reaction
answer
a chemical reaction that absorbs more energy than they release.
question
pH
answer
measures a solutions acidity or alkalinity.
question
Homeostatic control pH of blood
answer
7.35-7.45
question
death occurs between what numbers on pH scale?
answer
less than 6.8 and greater than 7.8
question
If a solution a low pH it means it is _______
answer
acidic
question
If a solution has a high pH it means it is _______
answer
alkaline
question
A solution that is acidic has a higher amount of _________
answer
hydrogen+
question
A solution that is alkaline has a higher amount of _________
answer
OH (-) hydroxide ions
question
A change of one whole number on the pH scale represents a tenfold change in the number of H ions.
answer
ph of 4 means that a solution contains 1x10^(-4) of a molecule of Hydrogen ions per liter ph of 8 means that a solutions contains 1x10^(-8) of molecules of hydrogen ions per liter
question
Buffer System
answer
A group of chemicals in the body that work together to maintain normal pH levels in tissues and body fluids by removing or adding H+.
question
What is a very important buffer system in the body (blood)?
answer
Carbonic Acid-Bicarbonate buffer system.



