Chemistry Topic 6 – Bonding
Unlock all answers in this set
Unlock answersquestion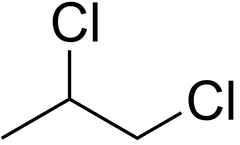
Asymmetric molecule

answer
A molecule that lacks identical atomic structure on each side of an axis.
question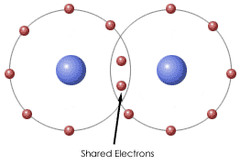
Covalent bond

answer
Type of bond formed when two nuclei share electrons in order to achieve a stable arrangement or electrons.
question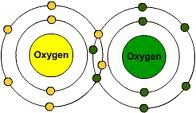
Double covalent bond

answer
Type of multiple covalent bond when two pairs of valence electrons are shared.
question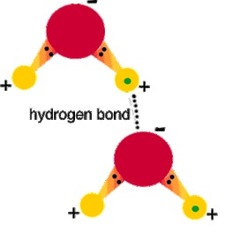
Hydrogen bond

answer
An intermolecular bond formed between a hydrogen atom in one molecule and a nitrogen, oxygen, or fluorine atom in another molecule.
question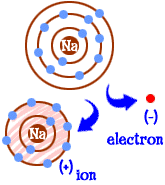
Ion

answer
Atoms that have gained or lost electrons and have become charged particles.
question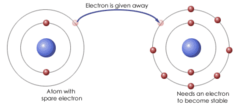
Ionic bond

answer
A bond formed by the transfer of electrons from one atom to another.
question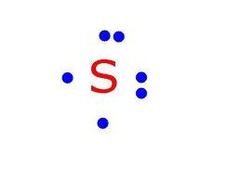
Lewis dot diagram/ electron dot diagram

answer
Consists of a chemical symbol surrounded by one to eight dots representing valence electrons.
question
Malleability

answer
The ability of metals to be hammered into shapes.
question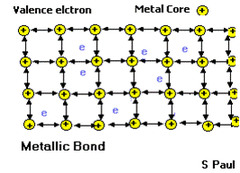
Metallic bond

answer
Results from the force of attraction of the mobile valence electrons for an atom's positively charged kernel.
question
Multiple covalent bond
answer
Type of covalent bond in which atoms share more than one pair of electrons.
question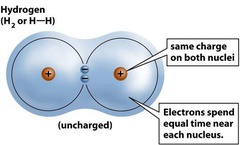
Nonpolar covalent bond

answer
Type of covalent bond when the attraction of two nuclei for the shared electron(s) is equal, causing the electron(s) to be shared equally.
question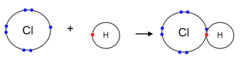
Octet

answer
The configuration of eight valence electrons.
question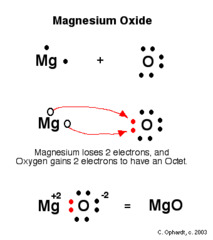
Octet rule

answer
States that atoms generally react by gaining, losing, or sharing electrons in order to achieve a complete octet of eight valence electrons.
question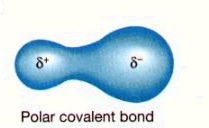
Polar covalent bond

answer
The unequal sharing of electrons in a covalent bond.
question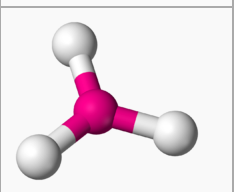
Symmetrical molecule

answer
A molecule with identical atomic structure on each side of the axis.
question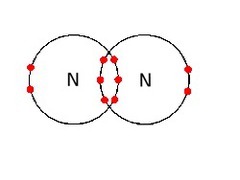
Triple covalent bond

answer
Type of multiple covalent bond in which three pairs of valence electrons are shared.



