CHEMISTRY TOPIC 1: THE ATOM REVIEW
Unlock all answers in this set
Unlock answersquestion
Rutherford Experiment:
answer
Bombarded gold foil with alpha particles. Showed atoms were mostly empty space with small, dense positively charged nucleus.
question
Bohr Model:
answer
Small, dense, positively charged nucleus surrounded by electrons in circular orbits.
question
Wave-Mechanical Model (Modern Atomic Theory)
answer
Small, dense, nucleus positively charged nucleus surrounded by electrons moving in "electron cloud". "Orbitals" are areas where an electron with a certain amount of energy is most likely to be found.
question
* Each atom is made of a......*
answer
positively charged nucleus with one or more orbiting, negatively charged electrons.
question
* Protons and neutrons are found in the *
answer
nucleus
question
Protons have a ...........charge
answer
positive
question
neutrons have.......... charge
answer
no
question
electrons have a.............charge
answer
negative
question
The mass of a proton is
answer
1 amu.
question
The mass of a neutron is
answer
1 amu
question
The mass of an electron is almost
answer
1/1836 amu
question
When all electrons are at their lowest possible energy, it is called the
answer
ground state
question
When the electron gains a specific amount of energy, it moves to a higher orbital and is in the
answer
"excited state".
question
When an electron returns from a higher energy state to a lower energy state, it ......
answer
emits a specific amount of energy usually in the form of light. This can be used to identify an element (bright line spectrum). The instrument used to see the bright line spectrum is called a spectroscope.
question
The outermost electrons are called valence electrons. These affect the..............................
answer
chemical properties of the element.
question
Atoms of the same element all contain the same number
answer
of protons
question
Isotopes are atoms with..............
answer
equal numbers of protons but different numbers of neutrons. *different atomic masses (protons + neutrons).*
question
The average atomic mass of an element is the
answer
weighted average of its naturally occurring isotopes.
question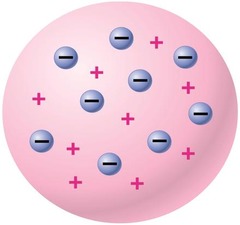
JJ Thomsons model

answer
Used cathode ray tube Negative electrons (in mixture ) Plum pudding model !!! -uniform density
question
When an atom is neutral ...
answer
Protons = electrons
question
Mass number
answer
Protons +neutrons
question
Atomic number
answer
# of Protons = # of electrons
question
Isotopes
answer
Same atomic number but different mass number - different number of neutrons
question
Calculating atomic mass
answer
% abundance x mass for each isotope (add all) 1 change percent to a decimal 2 multiply decimals with amu ( correct sigs 3 add all masses together ( sigs
question
Ion
answer
~ gain electrons = negative ion ~lose electrons = positive ion



