Chemistry Review-Water properties of water
Unlock all answers in this set
Unlock answersquestion
Which statement must be mentioned in explaining why amphipathic molecules line up at a water surface?
answer
Polar groups attract one another.
question
Dissolving is best described as

answer
mingling of molecules and/or ions.
question
Water is a source of ______________ for chemical reactions in cells.
answer
Oxygen and Hydrogen.
question
Which statement is true of water's tensile strength?
answer
(a) It results from hydrogen bonding. (b) It helps to pull water through plants. (c) It involves both cohesion and adhesion. Both (a) and (b). (a), (b), and (c).
question
Water has surface tension because
answer
hydrogen bonds between surface water molecules resist being stretched
question
Which of the following helps most to explain why water has a high specific heat?
answer
A water molecule can make 4 hydrogen bonds.
question
Which factor is important in making it possible to cool yourself by sweating?
answer
(a), (b), and (c). ; Random collisions allow some molecules to accumulate more energy than other molecules. The weakness of hydrogen bonds lets those molecules escape, leaving the cooler molecules behind.
question
Though you add heat, the temperature of boiling water remains constant because
answer
t takes energy to break hydrogen bonds.
question
Which statement helps to explain why ice is less dense than liquid water?
answer
-Water molecules make hydrogen bonds at definite angles. -Cold molecules move less than warm molecules.
question
The open spaces in water's crystal structure make it possible for
answer
aquatic life to exist at the North Pole
question
Why doesn't oil mix with water?
answer
Polar molecules attract one another.
question
Organizing Effects of water
answer
Life realist on the properties of water. Water organizes other kinds of molecules into structures and make up cells. Water organizes molecules on the basis of their polarity. In this respect, molecules fall into three categories; non polar, polar, and amphipathic. Water itself is highly polar.
question
Water
answer
An inorganic molecule composed of an oxygen atom covalently bound to two hydrogen atoms (H2O)
question
Molecules

answer
Two or more atoms held together by chemical bond.
question
Cell

answer
The smallest unit of matter that has all the properties of life, including the ability to maintain and reproduce itself and to conduct metabolism. All organisms consist of one or more cells. The human body contains trillions of cells. (Picture animals cell)
question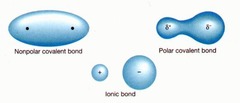
Non-polar molecule

answer
Having little or no partial charge on the component atoms. Single pair of bonded atoms or to a molecule as a whole. Bonds are non polar when they join atoms of similar electronegativity, as in bonds between C and H or between two C atoms or two O atoms.
question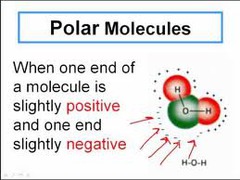
Polar molecule

answer
In a molecules or bonds, the presence of distinct regions that have partial positive and negative charges.
question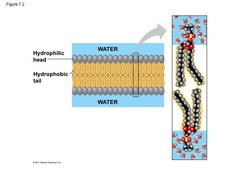
Amphipathic molecule

answer
Having both polar and non polar parts. Water causes amphipathic molecules to arrange themselves so their polar parts are in the water and their non polar parts are excluded from the water, as in biological membranes and proteins. Thus, amphipathy is fundamental to the structure of life.
question
Oil Molecules
answer
Oil molecules are non polar and cannot form hydrogen bonds. To break into a water mass, they must force water molecules apart, breaking hydrogen bonds. That takes energy. If oil moles do get into water by our shaking the mixture, they form clumps and wander about until water expels them.
question
More Polar Less polar
answer
Dissolves in water easily. (Methanol) Dissolves in water less easily. (Myristic Alcohol) (Butanol: middle)
question
Do molecules break apart when they dissolve?
answer
In water, some molecules break into ions, but most do not. For instance, a sugar cube contains many molecules held together by hydrogen bonds. When a sugar cube dissolves, each molecule stays intact. It trades the old hydrogen bonds for new ones with water. Salt, on the other hand, doesn't consist of molecules; it's just ions that separate water.
question
Does water repel non polar molecules:
answer
There is no repulsion between water and non polar molecules. The two don't mix simplify because water molecules attract one another and won't make room for molecules that lack attractive charged regions. When water expels a non polar molecule, it's because the water molecules pull one another together behind departing non polar molecule.
question
Does water treat all polar molecules alike?
answer
Not at all. Highly polar moles get into water more easily than less polar ones. Molecules vary widely in polarity depending partly on the balance between non polar and polar parts.
question
Chemical Reactions with Water
answer
Water provides hydrogen (H) and (O) atoms for making biological molecules. This plant is conducting photosynthesis. using the O from water to make atmospheric oxygen (O2), ad combining the H from water with carbon dioxide (CO2) to make sugar (C6H12O6). Many other reactions transfer OH from water to biological molecules. This happens when we digest proteins and starch.
question
Hydrogen (H)

answer
The chemical element composed of atoms that have 1 proton in the nucleus; symbolized by the letter H. The smallest and lightest atom, one of the four most abundant elements in biological molecules, and the most abundant element in the universe.
question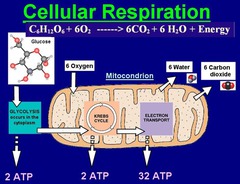
Oxygen O2

answer
The chemical element composed of atoms that have 8 protons in the nucleus; symbolized by the letter O. One of the four most abundant element in biological molecules, and the most abundant element ( on a weight basis) in Earths crust, water, and atmosphere.
question
Photosynthesis

answer
The metabolic pathway by which light energy is used to combine carbon dioxide and water into sugar and molecular oxygen.
question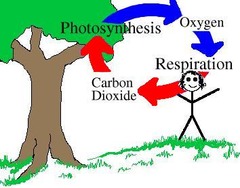
Carbon Dioxide

answer
Two oxygen atoms bound to one carbon atom by double bonds; CO2. This simple, low-energy compound is life's principal source of carbon. Organisms release it into the air as they process fuels for energy. Some organisms, chiefly plants, convert CO2 back to sugars with the air of light energy (the process of photosynthesis).
question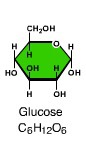
Sugar (C6h12O6)

answer
An organic molecule that has the formula CnH2nOn, and that has at least one O atom bound to each C atom. Sugars are the simplest carbohydrates.
question
Tensile Strength of water
answer
Water evaporates from leaves, which stay moist by pulling water up from roots against the pull of gravity. This stretches the water in the tree, but the water molecules resist breaking apart because of tensile strength. Tensile strength results from hydrogen bonds that link water molecules to one another (cohesion) and to cell walls (adhesion)
question
Tensile Strength

answer
The ability of a material to resist breaking when stretched.
question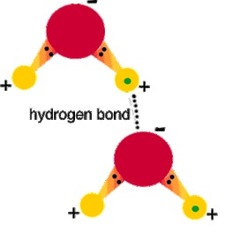
Hydrogen Bond

answer
An electric attraction that occurs when a hydrogen atom with a partial positive charge comes near another atom (typically O or N) with partial negative charge. The partial charges derive from the atoms being engaged in covalent bonds with aims that differ great in electronegativity.
question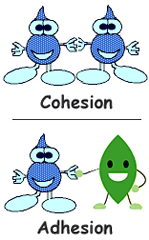
Cohesion

answer
The binding together of like molecules, often by hydrogen bonds.
question
Cell wall

answer
A protective layer external to the plasma membrane in plant cells, bacteria, fungi, and some protists. In the case of plant cells, the wall is formed of cellulose fibers embedded in a polysaccharide-protein matrix. The primary cell wall is thin and flexible, whereas the secondary cell wall is stronger and more rigid, and the primary constituent of wood.
question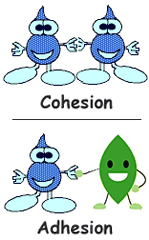
Adhesion

answer
The tendency of different kinds of molecules to stick together.
question
How high can tress lift water
answer
The tallest tress (pacific coast redwoods) lift water over 100 meters (365ft) against the pull of gravity. Laboratory experiments suggest that water has enough tensile strength to be pulled to the tops of tress three times that heights.
question
Why do paper towels absorb water?
answer
Paper towels are made of molecules from plant cell walls, which form hydrogen bonds with water. As randomly moving water molecules enter the towel, they adhere to the towel molecules, and cohesion pulls more water molecules after them. All these events forces result from hydrogen bonds.
question
What does adhesion contribute to tensile strength?
answer
Adhesion maintains the width of water mass by holding water to cell walls (or, in pipes, to the metal of the pipe). Without that effect, any force pulling on the water would make the water mass thinner and thinner until it's so narrow that it breaks.
question
Surface tension of Water
answer
The cohesion of water creates a force called surface tension that resists the entry of water-repellent objects such as this water strider. To overcome surface tension, a non polar object must push hard enough to break many hydrogen bonds. Water striders don't weigh enough to exert such force. This means little to us, but can be vital to a tiny insect.
question
Surface Tension

answer
A measure of how difficult it is to stretch or break the surface of a liquid. Water has a high surface tension because of the hydrogen bonding of surface molecules.
question
Non-polar

answer
Having little or no partial charge on the component atoms. The term can apply to a single pair of bonded atoms or to a molecule as a whole. Bonds are non polar when they join atoms or similar electronegativity, as in bonds between C and H or between two C atoms or two O atoms.
question
Hydrogen Bond

answer
An electric attraction that occurs when a hydrogen atom with a partial positive comes near another atom (typically O or N) with a partial negative charge. The partial charges derive from the atoms being engaged in covalent bonds with atoms that differ greatly in electronegativity.
question
Why don't all small objects float on water?
answer
Even if it's small, an object will sink into water if it concentrates enough weight on a small area (as in the point of a needle). With such a concentration, there aren't enough hydrogen bonds to resist the force. Also, an object will sink if it can make hydrogen bonds and soak up water, as with paper.
question
High specific heat of water
answer
With its oceans, Earth has a milder climate than a dry planet. You see the same effect in comparing seacoasts with the desert. This is due to water's high specific heat--water can gain or lose heat with little change in temperature, because of the heat is used to break hydrogen bonds. At night, hydrogen bonds return, releasing heat so the temperature does not drop far.
question
Specific heat

answer
The amount of heat that must be absorbed or lost for 1g of a substance to change its temperature 1C.
question
How does water's specific heat affect organisms
answer
Stored water, as in these cacti, helps to stabilize an organism's temperature, so it doesn't rise as far during the day or fall as far at night. This occurs because water has a high specific heat--that is, much heat can be gained or lost with only a small change in temperature. This results from hydrogen bonding in water.
question
How does heat relate to temperature
answer
Heat is the total kinetic energy stored in random motion of atoms or molecules. Two cups of boiling-hot tea have twice as much heat energy as one cup. Temperature measures the heat energy of the aerate atom or molecule. It's same for two cups of tea as for one cup, because its an average rather than a total.
question
Why do hydrogen bonds cause high specific heat
answer
much of the heat absorbed by water is used to break hydrogen bonds. only a small amount of heat is left over to increase molecular motion. Since temperature measures molecular motion, the temperature rises only slightly. Molecules that don't make hydrogen bonds, like CO2, attract each other more weakly , so a larger fraction absorbed heat can raise temperature.
question
Cooling by Evaporation
answer
Sweating cools you off because the fastest-moving (hottest) water molecules break every hydrogen bond and escape, carrying away heat. The escape is evaporation, so this way of losing heat is called evaporating cooling. Plants and many animals cool themselves in this way. It can be dangerous if too much water is lost, but it's worth the risk if the alternative is fatal overheating.
question
Evaporative cooling

answer
The property of a liquid whereby the surface becomes cooler during evaporation, owing to a loss of high kinetic molecules to the gaseous state.
question
What gives some molecules more energy?
answer
When two molecules collide and rebound, one molecule may give energy to the other. Because collisions occur at varied speeds and angles, the average molecule gains energy as often as it loses energy. But sometimes, by chance, a molecule gains energy in several successive collisions, giving it much more energy than the average molecule.
question
Boiling
answer
Boiling occurs when all the water molecules have so much energy that their motion break most of the hydrogen bonds. Then the liquid rapidly changes to gas bubbles (steam). If you keep adding heat to boiling water, the temperature stays the same because all the heat is used to break hydrogen bonds. The free molecules (steam) carry away energy.
question
Freezing

answer
If the temperature drops below about 0c (The freezing point), water changes from liquid to a crystalline solid (ice). With too little energy to break hydrogen bonds very often, each water molecule forms four hydrogen bonds that pull the molecules into a regular arrangement (a lattice).
question
Crystalline

answer
A group of atoms or molecules that are uniformly arranged in space.
question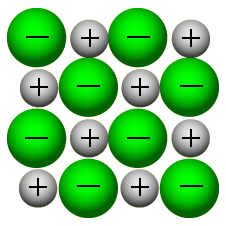
Lattice

answer
A regular arrangement of points in space, marking the positions where particular atoms or molecules occur in a crystal.
question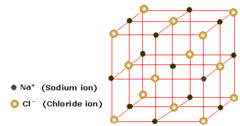
What's a lattice

answer
A lattice is a regular arrangement of points in space. In ice, each oxygen atom of water molecule occurs at a point of the lattice. The water molecules are held in place by hydrogen bonds.
question
Can Ice evaporate?
answer
When it's very cold, ice may evaporate without melding. This change from solid ice directly to gaseous vapor is called sublimation. sublimation occurs because even in the cold, molecules occasionally gain enough energy in collisions without molecules to break away from the solid ice lattice.



