Chem105 Ch. 1-2 – Flashcards
Unlock all answers in this set
Unlock answersquestion
The air we breathe is a... a. Element b. Mixture c. Compound d. Pure substance
answer
b. mixture
question
Ozone is considered an air pollutant in the ______ and is a valuable protective layer in the _______.
answer
Troposphere; stratosphere
question
The EPA limit for CO is 9 ppm. Express this number as a percentage.
answer
0.0009%
question
How to convert from ppm to percentage?
answer
Move decimal four places to the right. So 5 ppm would become 0.0005%
question
What two factors are considered when determining the risk assessment for air pollutants? a. toxicity and exposure b. percentage and ppm c. toxicity and percentage d. exposure and ppm
answer
a. toxicity and exposure
question
In general, which airborne material is not likely to be captured by the filters of indoor air handling equipment? a. particulates b. carbon monoxide c. soot d. pollen
answer
b. carbon monoxide
question
Which two gases make up more than 95% of an inhaled breath?
answer
O2 and N2
question
What is the composition of the air we breathe?
answer
78% nitrogen 21% oxygen 1% other gases like hydrogen argon, carbon dioxide, methane
question
The most numerous of the elements in the periodic table are the... a. metals b. non-metals c. metalloids d. noble gases
answer
a. metals
question
As the ozone hole gets more pronounced, with time, one expects the incidence of skin cancer to
answer
increase worldwide
question
The _____ concentration in the air over the desert differs dramatically from that in the air in the tropical rainforest a. H2O b. O2 C. CO2 D. N2
answer
a. H2O
question
If we assume that the top of Mt. Everest is the highest land mass on earth, hikers who scale its summit are standing in the
answer
Troposphere (still the zone closest to earth)
question
What substance is an element? a. NO2 b. NaCl c. CH4 d. N2
answer
D. N2
question
What classifies a molecule vs. a compound?
answer
A molecule is formed when two or more atoms join together chemically. (ex. H2O or N2) A compound is a molecule that contains at least two different elements. (H2O, but not N2) All compounds are molecules but not all molecules are compounds.
question
The chemical formula for nitrogen monoxide is... A: N2O2 B: N2O C: NO2 D: N2O3
answer
B. N2O
question
P2O5 is the chemical formula for
answer
diphosphorus pentoxide
question
During a chemical reaction, a. atoms are simply rearranged b. some atoms are destroyed and new ones are formed. c. some elements are destroyed and new ones are formed. d. the law of conservation of matter and mass may be briefly violated
answer
a. atoms are simply rearranged
question
What are the usual reactants and products of a combustion reaction?
answer
Hydrocarbon reacts with oxygen to produce water and carbon dioxide
question
Catalytic converters reduce the amount of _____ in car exhaust. a. CO b. N2 c. O3 d. CO2
answer
a. CO
question
What do catalytic converters do?
answer
??
question
Which correctly pairs an indoor pollutant with its source? a. O3 and electrical arcing b. Formaldehyde and unvented space heaters c. nicotine and paint and paint thinners d. radon and glues and solvents
answer
a. O3 and electrical arcing
question
The two main products of the combustion of gasoline in an automobile engine are... a. oxygen and carbon monoxide b. Water and carbon dioxide c. sulfur oxides and hydrogen d. sulfur oxides and nitrogen oxides
answer
b. water and CO2 (carbon dioxide)
question
HFCs may be used to replace CFCs. Which compound is a HFC? a. CH2CL-CCl2F b. CH2FCl c. CF3CH2F d. CHClF2
answer
c. CF3CH2F
question
Which contributes to the ozone hole? a. automobile exhaust b. loss of Northern forests c. chlorofluorocarbons (CFCs) d. all of these choices are correct
answer
c. chlorofluorocarbons (CFCs)
question
You wear sunscreen on your skin in order for the sunscreen to _________, thereby protecting your skin from some of the sun's radiation. a. absorb UV-A and UV-B radiation b. reflect visible radiation and uv-b radiation c. Transmit UV-A and UV-B radiation d. Reflect infrared radiation
answer
a. absorb uv-A and UV-B radiation
question
The speed of light in air a. depends only on the frequency of the light b. depends only on the wavelength of the light c. is independent of the wavelength and frequency of light d. depends on both the wavelength and the frequency of light
answer
c. is independent of the wavelength and frequency of light
question
Which is/are part of the Chapman Cycle in the stratosphere? a. Ozone is removed by its reaction with water vapor b. ozone is removed by an interaction with UV radiation c. Ozone reacts with oxygen atoms to form oxygen molecules. d. ozone is removed by an interaction with UV radiation and it reacts with oxygen atoms to form oxygen molecules
answer
d. ozone is removed by an interaction with UV radiation and it reacts with oxygen atoms to form oxygen molecules
question
The goal of the Montreal Protocol in 1987 was to... a. reduce the amount of new production of CFCs in developed countries b. recycle existing CFCs rather than release them into the air c. encourage research into substitutes for CFCs d. all of these are correct
answer
d. all of these are correct
question
Which color in the rainbow has the longest wavelength? a. red b. orange c. yellow d. blue
answer
a. red
question
How many protons, neutrons and electrons are in a neutral atom of Fluorine? 19 F 9

answer
Protons - 9 Neutrons -10 Electrons- 9 (19 is the atomic mass number, or P + N)
question
In the atmosphere over the earth, where is the region with the highest concentration of ozone? a. troposphere b. biosphere c. mesosphere d. stratosphere
answer
d. stratosphere
question
Chlorofluorocarbons rise to the stratosphere and a. react directly with stratospheric ozone b. interact with UV energy to produce free radicals c. interact with UV energy to produce free radicals that react with oxygen to create ozone d. react with free radicals to remove carbons dioxide
answer
b. interact with UV energy to produce free radicals that destroy ozone (thus creating the ozone hole)
question
Yellow light has a wavelength of 580 nm. What is the frequency of this light? a. 2.39 x 10 ^-19 b. 1.8 x 10 ^-7 c. 5.17 x 10 ^5 d. 5.17 x 10 ^14
answer
3 x 10 ^ 8 m/s / 580 x 10 ^ -9 m = c. 5.17 x 10 ^ 14 s-1
question
When dividing numbers that are written in exponential form, the coefficients are _____ and the exponents are ____
answer
Divided, subtracted
question
Decreasing wavelength of light goes in this order (longest to shortest:) a. UV - visible - infrared b. Visible - infrared - UV c. UV- infrared - visible d. infrared - visible - UV
answer
d. infrared - visible - UV
question
DNA, the genetic material of living organisms, is damaged by light in the a. visible region of the spectrum b. infrared region of the spectrum c. UV region, especially above a wavelength of 340 nm d. UV region, especially below a wavelength of 320 nm
answer
d. UV region, especially below a wavelength of 320 nm
question
Wavelength is the...
answer
distance between the successive peaks in a wave
question
O2 and O3 molecules are
answer
allotropes
question
UV-B radiation has a frequency of approximately 10 ^ 17 s-1 (Hz). What is the energy of a photon of this light? (Hint: h = 6.63 x 10 ^ -34 J s)
answer
6.63 x 10 ^-17 J because E = Planck's (h) x frequency so E = (6.63 x 10 ^-34)(
question
Which is the third most abundant gas in the air we breath?
answer
Argon
question
HCFCs, the current CFC replacement, is preferred over CFCs because
answer
c. They decompose in the troposphere
question
The Montreal Protocol took several approaches to reducing stratospheric chlorine. CFC production and emissions dropped to very low levels once all countries ratified it and yet an ozone hole still remains. Why? a. CFCs are catalysts to the ozone depletion process and remain in the stratosphere for long periods of time b. The USA still has not ratified the Montreal Protocol c. CFCs are naturally occurring and so continue to deplete ozone d. Natural events such as lightning deplete ozone
answer
a. CFCs are catalysts to the ozone depletion process and remain in the stratosphere for long periods of time The chlorine atoms act as a catalyst, and each can break down tens of thousands of ozone molecules before being removed from the stratosphere. Given the longevity of CFC molecules, recovery times are measured in decades. It is calculated that a CFC molecule takes an average of about five to seven years to go from the ground level up to the upper atmosphere, and it can stay there for about a century, destroying up to one hundred thousand ozone molecules during that time.
question
2. Which is not a mixture? a. a jar filled with rocks and sand b. sea water c. a glass of Kool-Aid d. sodium chloride
answer
d. sodium chloride
question
Which color in the rainbow has the shortest wavelength a. orange b. red c. yellow d. blue
answer
d. blue
question
The wavelength of light in the visible range is a. about the size of an atom of carbon b. intermediate between the size of an animal cell and a virus c. about the diameter of a CD d. intermediate between the size of an animal cell and the diameter of a CD
answer
b. intermediate between the size of an animal cell and a virus
question
Which statement is correct? a. UV-A is the most energetic of the three forms of UV light b. UV-B is the most energetic of the three forms of UV light c. UV-C is the most energetic of the three forms of the UV light d. UV-A, UV-B, and UV-C are equally energetic
answer
c. UV-C is the most energetic of the three forms of the UV light
question
During the Antarctic spring, ozone is destroyed at a greater rate than it is formed a. on the surface of atmospheric crystals b. in polar stratospheric clouds c. in a process that is catalytic d. all of these choices are correct
answer
d. all of these choices are correct
question
The ozone hole is most prominent on the Earth over a. North America b. Europe c. Africa d. Antartica
answer
d. Antartica
question
Ozone in our atmosphere is important because it a. absorbs some UV radiation b. reacts with excess CO2 c. helps trees grow d. reflects IR radiation
answer
a. absorbs some UV radiation
question
The Montreal protocol is a a. treaty to protect against global warming b. treaty to reduce the amount of CFCs produced in the world c. list of substitutes for CFCs d. way to destroy CFCs in the stratosphere
answer
b. treaty to reduce the amount of CFCs produced in the world
question
What is the relationship between stratospheric levels of atomic chlorine and ozone? a. As chlorine increases, ozone increases b. as chlorine increases, ozone decreases c. as chlorine changes, the effect on the ozone level is unpredictable d. As chlorine changes, there is no effect on the ozone level
answer
b. as chlorine increases, ozone decreases
question
In the periodic table, which elements typically have similar properties? a. those in the same row b. those in the same columns c. those related diagonally d. those on opposite sides
answer
b. those in the same columns
question
In the atmosphere over the Earth, where is the region with the highest concentration of ozone? a. troposphere b. biosphere c. mesosphere d. stratosphere
answer
d. stratosphere
question
The nucleus of an atom contains a. electrons and protons only b. electrons, protons, and neutrons c. protons only d. protons and neutrons only
answer
d. protons and neutrons only
question
What distinguishes the atoms of one element from another? a. number of neutrons b. number of protons c. number of protons plus neutrons d. number of neutrons plus electrons
answer
b. number of protons
question
Elements in the same column of the periodic table in the groups labeled A tend to have similar chemical and physical properties because they have the same number of a. valence electrons b. protons c. protons plus electrons d. protons plus neutrons
answer
a. valence electrons
question
Isotopes of an element have the same number of ____ but a different number of ____ a. electrons/protons b. protons/neutrons c. neutrons/protons d. protons/electrons
answer
b. protons/neutrons
question
When only one pair of shared electrons is involved in a covalent bond, the linkage is called a _____ bond. a. triple b. single c. double d. resonant
answer
b. single
question
The atomic number is the a. same as the mass number of an atom b. number of protons and neutrons in a nucleus c. number of protons in a nucleus d. number of neurtons in a nucleus
answer
c. number of protons in a nucleus
question
The periodicity of the properties of element is chiefly due to a. the numbers of electrons in the atoms of the elements b. the distribution of electrons in the atoms of the elements c. the numbers of neutrons and electrons in the atoms of the elements d. both the numbers of electrons in the atoms of the elements and the distribution of electrons in the atoms of the elements
answer
d. both the numbers of electrons in the atoms of the elements and the distribution of electrons in the atoms of the elements
question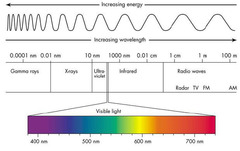 visible> infrared b. infrared> visible> ultraviolet c. Visible> infrared> ultraviolet d. ultraviolet> infrared> visible" alt="The wavelength of light goes in this order a. Ultraviolet> visible> infrared b. infrared> visible> ultraviolet c. Visible> infrared> ultraviolet d. ultraviolet> infrared> visible">
visible> infrared b. infrared> visible> ultraviolet c. Visible> infrared> ultraviolet d. ultraviolet> infrared> visible" alt="The wavelength of light goes in this order a. Ultraviolet> visible> infrared b. infrared> visible> ultraviolet c. Visible> infrared> ultraviolet d. ultraviolet> infrared> visible">
The wavelength of light goes in this order a. Ultraviolet> visible> infrared b. infrared> visible> ultraviolet c. Visible> infrared> ultraviolet d. ultraviolet> infrared> visible
 visible> infrared b. infrared> visible> ultraviolet c. Visible> infrared> ultraviolet d. ultraviolet> infrared> visible" alt="The wavelength of light goes in this order a. Ultraviolet> visible> infrared b. infrared> visible> ultraviolet c. Visible> infrared> ultraviolet d. ultraviolet> infrared> visible">
visible> infrared b. infrared> visible> ultraviolet c. Visible> infrared> ultraviolet d. ultraviolet> infrared> visible" alt="The wavelength of light goes in this order a. Ultraviolet> visible> infrared b. infrared> visible> ultraviolet c. Visible> infrared> ultraviolet d. ultraviolet> infrared> visible">answer
b. infrared> visible> ultraviolet (UV has shorter waves than infrared)
question
The wavelength of light in the X-ray region of the electromagnetic spectrum is a. smaller than a virus b. about the size of a bacterial cell c. intermediate between the size of a bacterial cell and a virus d. larger than either a bacterial cell or a virus
answer
A: smaller than a virus.
question
Stratospheric ozone is destroyed and formed at the same rate a. above the equator b. above the Antarctic in its early spring c. above the Antarctic in its early fall d. above the equator and above the Antarctic in its early fall
answer
d. above the equator and above the Antarctic in its early fall.
question
The mass number of an isotope of an element is the a. sum of the number of its protons and electrons b. number of its protons c. sum of the number of its protons and neutrons d. sum of the number of its protons, neutrons, and electrons
answer
c. sum of the number of its protons and neutrons
question
it is the ___ electrons that account for many of the chemical and physical properties of elements a. innermost b. intermediate c. outermost d. transitional
answer
c. outermost
question
Single bonds, double bonds, and triple bonds a. have 1, 2, 3 shared electrons respectively b. have 2, 4 and 6 shared electrons respectively c. have 3, 6, and 9 shared electrons respectively d. are only possible between carbon atoms
answer
b. have 2, 4 and 6 shared electrons respectively
question
b Light behaves like a. a particle b. a wave c. both a particle and a wave d. neither a particle nor a wave
answer
c. both a particle and a wave
question
The "ozone layer" is found a. only around the equator b. in the stratosphere c. in the troposphere d. in the mesosphere
answer
b. in the stratosphere
question
In references to waves, frequency is the a. number of waves passing a fixed point in one second b. height of the wave c. distance between successive peaks in a wave d. distance between a peak in a wave to the next trough
answer
a. number of waves passing a fixed point in one second (c is wavelength)
question
Free radicals are a. highly reactive chemical species b. species such as H and OH c. species with unpaired electrons d. ALL of these correctly describe free radicals
answer
d. ALL of these correctly describe free radicals
question
Chlorofluorocarbons rise to the stratosphere and a. react directly with stratospheric ozone to destroy it b. interact with UV energy to produce free radicals that destroy ozone c. interact with UV energy to produce free radicals that react with oxygen to create ozone d. react with free radicals to remove carbon dioxide
answer
b. interact with UV energy to produce free radicals that destroy ozone
question
Halons differ from CFCs in that the atoms of ___ replace some____ atoms. a. iodine/chlorine b. hydrogen/chlorine c. bromine/chlorine d. silicon/carbon
answer
c. bromine/chlorine
question
Which region of the ultraviolet spectrum is absorbed least by the atmosphere? a. UV-A b. UV-B c. UV-C
answer
a. UV-A
question
Which product of ultraviolet decomposition of CFCs acts as the catalyst for ozone decomposition? a. oxygen atoms b. chlorine atoms c. fluorine atoms d. hydrogen atoms
answer
b. chlorine atoms
question
HCFCs have been developed to replace CFCs as refrigerants. Which property of these new compounds make them environmentally superior to CFCs a. Greater reactivity leads to decomposition at elevations below the stratospheric ozone concentration maximum b. lower reactivity makes them stable even in the intense ultraviolet light in the stratosphere c. Their higher molecular weight prevents them from reaching the stratosphere d. they do not contain chlorine
answer
a. Greater reactivity leads to decomposition at elevations below the stratospheric ozone concentration maximum Explanation: CFCs are mainly stable compounds at low altitudes, but are lighter than air. As the compounds travel to the upper atmosphere, UV radiation breaks off the chlorine molecule. This chlorine then binds with ozone molecules, causing them to break apart. Though HFCs also impact the environment, their effect is considered mild due to the short life spans of HFC particles.
question
HCFCS are temporary solution to the problem of ozone depletion and will be replaces over the next 20 years by which class of compounds a. HFCs b. CFCs. d. Halons d. HFBCs
answer
a. HFCs
question
Why are HFCs environmentally superior to the currently used HCFCs a. HFCs are not flammable b. HFCs do not contain chlorine c. HFCs are lighter and may be transported more easilty d. HFCs are less reactive than HCFCS
answer
b. HFCs do not contain chlorine
question
CFCs were originally developed to replace which refrigerant compounds a. ice b. HCFCs c. ammonia and sulfur dioxide d. propane
answer
c. ammonia and sulfur dioxide
question
which is correct? a. Ozone forms by combining an oxygen atom with an oxygen molecule b. there is a dynamic steady state of ozone in the stratosphere c. UV radiation will dissociate ozone into an oxygen atom and an oxygen molecule d. all of these choices are correct
answer
d. all of these choices are correct
question
11. The production of which of the following classes of compounds was NOT limited by the Montreal Protocol of 1987 nor by its amendments: A. CFCs B. HCFCs C. VOCs D. Halons
answer
C. VOCs
question
12. What is the primary component of an exhaled breath? A. N2 B. O2 C. CO2 D. H2O
answer
A. N2
question
25. Which is not a component used to determine an individual's exposure to a pollutant? A. length of contact time B. concentration of pollutant in the air C. toxicity D. total amount (volume) of air inhaled
answer
C. toxicity
question
28. Which pollutant could not be detected by its odor? A. CO B. O3 C. SOx D. NOx
answer
A. CO
question
29. Which characteristic describes a compound but not a mixture? A. Two or more things combined. B. The combining ratio is fixed. C. Substances can be broken down into smaller parts. D. Elements are combined.
answer
B. The combining ratio is fixed.
question
30. Chlorofluorocarbons rise to the stratosphere and A. react directly with stratospheric ozone to destroy it. B. after interacting with UV energy, become free radicals, which destroy ozone. C. become free radicals that react with oxygen to create ozone. D. react with free radicals to remove carbon dioxide.
answer
B. after interacting with UV energy, become free radicals, which destroy ozone.
question
45. Which approach would reduce indoor air pollution? A. air conditioning B. sealing windows shut C. increasing the air exchange D. dry cleaning clothes
answer
C. increasing the air exchange
question
Of the five major gaseous components of air, which is the only one to vary significantly in concentration from place to place and from day to day a. water vapor b. carbon dioxide c. nitrogen d. argon
answer
a. water vapor
question
Which component of the air makes up approximately 100 times more of an exhaled breath than of an inhaled breath? a. Ar b. O2 c. O3 d. CO2
answer
d. CO2
question
Which substance is not considered to be an air pollutant? a. N2 b. SO2 c. NO2 d. O3
answer
a. N2
question
What is the appropriate concentration unit used to express the concentration of a pollutant that has a concentration of 0.00004%? a. pph b. ppm c. ppb d. ppL
answer
b. ppm
question
When assessing the risk of an air pollutant, which does not play a role in considering someone's exposure to the pollutant? a. a persons's lung capacity b. the toxicity of the pollutant c. a person's breathing rate d. the concentration in air of the pollutant
answer
b. the toxicity of the pollutant Toxicity is used to create the limit, not to measure the exposure
question
The burning of coal produces sulfur dioxide, a pollutant that slowly reacts in air to form sulfur trioxide that dissolves into airborne water droplets to from a very corrosive solution of sulfiric acid. Which is a product of burning coal that hastens the transformation of sulfur dioxide into sulfur trioxide? a. carbon dioxide b. carbon monoxide c. nitrogen dioxide d. particles of ash
answer
d. particles of ash
question
Which pollutant are you more likely to encounter in dangerous concentrations indoors rather than outdoors? a. nitrogen dioxide b. carbon monoxide c. ozone d. sulfur dioxide
answer
b. carbon monoxide
question
Which color, as used in the Air Quality Index, warns that the level of a pollutant is hazardous, the most dangerous level? a. orange b. green c. yellow d. maroon
answer
d. maroon
question
A substance that can be broken down into two or more simpler substances by chemical methods is called an a. compound b. mixture c. element d. isotope
answer
a. compound CHEMICAL methods
question
On a periodic table, the columns of elements with similar properties are a. periods b. groups c. rows d. metals
answer
b. groups
question
which is not a single pure substance? a. copper b. water c. brass d. chlorine
answer
c. brass
question
Which differentiates a compound from a mixture of two or more elements? a. the elements in a compound may be present in varying proportions b. a compound does not exhibit the individual properties of the element of which it is composed c. a compound is made up of only one element d. a compound cannot be made up of more than two elements
answer
b. a compound does not exhibit the individual properties of the element of which it is composed (it is a new chemical substance)
question
A ____ is a fixed number of atoms held together by chemical bonds in a certain spatial arrangement a. element b. ion c. molecule d. compound
answer
c. molecule
question
A compound and a mixture differ in that only the mixture a. is made up of more than one element b. may be made up of components that may be molecules c. is of fixed composition d. exhibits the properties of its individual components
answer
d. exhibits the properties of its individual components`
question
Based on its name, which carbon compound contains the fewest carbon atoms? A: ethanol B: methane C: chlorobutane D: propyl alcohol
answer
B: methane
question
During a chemical reaction a. atoms are rearranged b. some atoms are destroyed and new ones are formed c. some elements are destroyed and new ones are formed d. the law of conservation of matter and mass may be briefly violated
answer
a. atoms are rearranged
question
Although only about 100 elements exist, over __________________ compounds have been isolated, identified, and characterized. ANSWERLIST A: one hundred thousand B: one million C: 20 million D: 10 billion
answer
C: 20 million
question
A young senator excited about Green Chemistry presents new legislation to set controls on vehicles and industry to completely remove all pollutant molecules from the air. Which is the best critique of this proposal? A: This worthy goal is attainable if we develop the proper incentives. B: Though this is a worthy goal, it is not attainable regardless of regulations or incentives. C: While this would be great in theory, accomplishing it would require too much money and more government control than is appropriate in a free market economy. D: This is not a good plan because while the United States concerns itself with this challenge, other countries will not make similar sacrifices.
answer
B: Though this is a worthy goal, it is not attainable regardless of regulations or incentives.
question
Critique the statement: "Air pollution is an unavoidable by-product of an industrialized society; the more modern we become the more air pollution we will have." A: This is a reasonable statement since burning fossil fuels is necessary for industrialization and unavoidably produces pollutants. B: Although burning fossil fuels does produce air pollutants, technology may prevent these pollutants from increasing in the atmosphere as we become more industrialized. C: Air pollution is not inevitable if we could raise the temperature at which fossil fuels are burned. D: Burning fossil fuels does not have to produce potential pollutants, but it does because of the unwillingness of government and industry to spend additional money.
answer
B: Although burning fossil fuels does produce air pollutants, technology may prevent these pollutants from increasing in the atmosphere as we become more industrialized.
question
The United States has more vehicles per capita than any other country, but cleaner air than many other countries because it A: has more electric vehicles. B: has fewer people living in cities. C: has more governmental regulations on air quality. D: is a large country so pollutants can spread out more.
answer
C: has more governmental regulations on air quality.
question
There are approximately 2 x 10^22 molecules and atoms in each breath we take and the concentration of CO in the air is approximately 9 parts per million. Approximately how many CO molecules are in each breath we take? A: 2 x 10^15 B: 1.8 x10^17 C: 2 x 10^17 D: 2 x 10^29
answer
C: 2 x 10^17
question
Which is correct? A: Ozone forms by combining an oxygen atom with an oxygen molecule. B: There is a dynamic steady state of ozone in the stratosphere. C: UV radiation will dissociate ozone into an oxygen atom and an oxygen molecule. D: All of these choices are correct.
answer
D: All of these choices are correct.
question
The structure of ozone most closely resembles a A: linear molecule with different lengths of chemical bonds B: linear molecule with the same length of chemical bonds C: bent molecule with different lengths of chemical bonds D: bent molecule with the same length of chemical bonds
answer
D: bent molecule with the same length of chemical bonds
question
When it reaches its largest size, the ozone hole over the Antarctic is A: about as large as North America. B: about the same size as Texas. C: smaller than Rhode Island. D: about the same size as California.
answer
A: about as large as North America.
question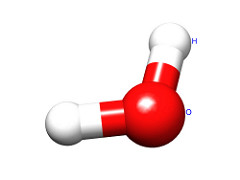
The two chemical bonds and geometry of water are best represented by:

answer
a bent molecule, with two atoms off the central atom
question
Decreased stratospheric ozone concentrations may lead to A: increased incidences of melanomas. B: a decreased production of crops such as wheat, sorghum, and peas. C: an increased occurrence of cataracts. D: All of these choices are correct.
answer
D: All of these choices are correct.
question
WUKF FM transmits at 93.5 MHz. What is the wavelength of the electromagnetic radiation that carries the station's signal?
answer
3.21 m wavelength x frequency = speed of light (so 3 x 10^8 / .935
question
How do you find wavelength?
answer
3x10^8 m/s = wavelength x frequency (planks constant speed of light) So... wavelength = 3 x 10^ 8 / frequency
question
Which region of the ultraviolet spectrum is absorbed least by the atmosphere? A: UV-A B: UV-B C: UV-C D: They are all absorbed approximately equally.
answer
A: UV-A
question
What is the difference between UV-A, UV-B, and UV-C?
answer
UV-C has the shortest wavelength and is the most damaging type of UV radiation. However, it is completely filtered by the atmosphere and does not reach the earth's surface. Medium-wavelength UVB is very biologically active but cannot penetrate beyond the superficial skin layers. It is responsible for delayed tanning and burning; in addition to these short-term effects it enhances skin aging and significantly promotes the development of skin cancer. Most solar UVB is filtered by the atmosphere. The relatively long-wavelength UVA accounts for approximately 95 per cent of the UV radiation reaching the Earth's surface. It can penetrate into the deeper layers of the skin and is responsible for the immediate tanning effect. Furthermore, it also contributes to skin ageing and wrinkling. For a long time it was thought that UVA could not cause any lasting damage. Recent studies strongly suggest that it may also enhance the development of skin cancers.
question
From 1974 to 2002, the chance that a white male would be diagnosed with melanoma skin cancer rose by
answer
225%
question
In the Chapman cycle, ozone formation depends upon a sufficient concentration of oxygen atoms. Which step in the Chapman cycle produces oxygen atoms? A: absorption of light wavelength 320 nm by ozone B: absorption of light wavelength 320 nm by oxygen C: absorption of light wavelength 242 nm by ozone D: absorption of light wavelength 242 nm by oxygen
answer
D: absorption of light wavelength 242 nm by oxygen
question
By approximately what percentage did global production of CFCs fall from 1987 to 2000? A: 13% B: 44% C: 88% D: 1100%
answer
c. 88%
question
Which product of the ultraviolet decomposition of CFCs acts as the catalyst for ozone decomposition? A: oxygen atoms B: chlorine atoms C: fluorine atoms D: hydrogen atoms
answer
B: chlorine atoms



