chem chapter 17 nuclear chemistry – Flashcards
Unlock all answers in this set
Unlock answersquestion
antione-henri-becquerel
answer
discovers radioactivity but called it "uranic rays" at first
question
marie curie
answer
finds out other elements emit radioactivity and got 2 nobel peace prizes in chemistry and physics
question
alpha radiation
answer
1. atom emits piece of itself 2. most potential to damage other cells 3. highest ionizing power 4. low penetrating power 5. slow speed and positive charge
question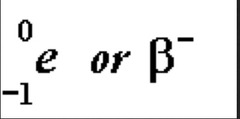
beta decay
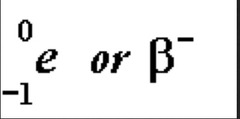
answer
1. neutron hits atom and neutron changes to proton 2. medium ionizing power and penetrating power 3.negative charge 4. no mass
question
gamma radiation
answer
1. electromagnetic radiation 2. high energy and short wavelength 3. usually happens with other types of decay 4. lowest ionizing energy 5. highest penetrating power 6. neutral charge
question
positron
answer
1. proton changes to neutron 2. medium penetrating power and ionizing energy
question
film badge dosimeter
answer
photographic film that measures radioactivity
question
Geiger counter
answer
particles pass through argon chamber and if voltage is high, a clicking noise is heard
question
scintillation counter
answer
radioactive emissions are passed through and if it is detected then it is turned to an electrical signal
question
half life
answer
time it takes for amount of atom to cut into half
question
when is an atom stable
answer
if ratio of protons to neutrons is 1:1
question
how do atoms gain stability
answer
go through decay
question
what part of an atom goes through decay
answer
nucleus
question
radiocarbon dating
answer
use carbon-14 to measure how old an organism is by using the half-life of carbon-14. the concentration of carbon-14 decreases when a living thing dies, it is constant when it is alive
question
radioactive decay
answer
atom loses part of itself to become stable
question
transmutation reaction
answer
reaction that changes nucleus, element turns to some other element
question
chain reaction
answer
atom keeps going through decay to become stable
question
band of stability
answer
a line that tells how stable atom is using neutrons/protons <1: positron 1: stable 1-1.49: beta 1.5<: alpha
question
radioactive isotope
answer
element that is unstable isotope of atom
question
what is the relationship between half-life and stabilitiy of the element
answer
as half-life increases atom becomes more stable
question
fission
answer
1. takes place in the nucleus 2. big thing breaks down into smaller things 3. element is hit with neutron 4. lots of energy
question
fusion
answer
1. small ones to bigger ones 2. used in sun 3. fusion has more energy that fission and is less dangerous 4. used in high temperature
question
what are properties of radioactive tracers
answer
have short half lives
question
how is radiation therapy different from tracers
answer
radiation therapy is more energy and used to treat cancer
question
isotope scanning
answer
patient is given isotope to identify cancer tumors which uses gamma rays and uses nuclear radiation
question
what body cells are most sensitive to ionization radiation
answer
reproductive organs becuase they divide fast and weak tissue cells
question
what type of radiation would most likely happen in your room
answer
gamma



