Chem 170 CH 0
Unlock all answers in this set
Unlock answersquestion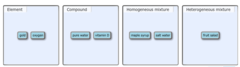

answer
Classify each of the following as an element, compound, homogeneous mixture, or heterogeneous mixture.
question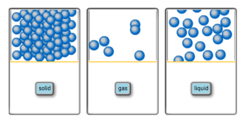

answer
The blue spheres below represent atoms. What state of matter is depicted in each bin?
question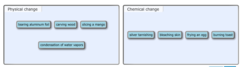
When tarnish forms on silver, the outer layer of silver atoms has reacted with sulfur to become a new substance, silver sulfide.

answer
Classify each of the changes as a physical change or a chemical change.
question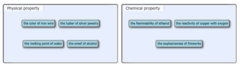

answer
Classify each of the properties as a physical property or a chemical property.
question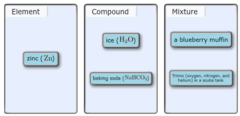

answer
Classify each of the following as an element, compound, or mixture.
question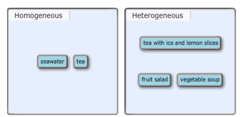

answer
Classify each of the following mixtures as homogeneous or heterogeneous.
question
Gas
answer
This substance has no definite volume or shape.
question
Gas
answer
The particles in this substance do not interact with each other.
question
Solid
answer
The particles in a substance are held in a rigid structure.
question

answer
What type of change, physical or chemical, takes place in each of the following?
question
Condensation
answer
The water vapor in the clouds changes to rain.
question
Evaporation
answer
Wet clothes dry on a clothesline.
question
Boiling
answer
Lava flows into the ocean and steam forms.
question
Condensation
answer
After a hot shower, your bathroom mirror is covered with water.
question
melting
answer
The solid structure of a substance breaks down as liquid forms.
question
sublimation
answer
Coffee is freeze-dried.
question
freezing
answer
Water on the street turns to ice during a cold wintry night.
question
deposition
answer
Ice crystals form on a package of frozen corn.
question
3
answer
Determine the number of significant figures in the measurement 6.07 m.
question
2 (Leading zeros are not significant. Trailing zeros are significant if there is a decimal point shown in the number.)
answer
Determine the number of significant figures in the measurement 0.0030 s.
question
45.0 g (The number 44.981 is closer to 45.0 than it is to 44.9.)
answer
Round the value 44.981 g to three significant figures.
question
16 (Because this calculation involved multiplication, the final answer must be rounded to the same number of significant figures as the measured value with the fewest significant figures.)
answer
Compute 3.5 × 4.48697. Round the answer appropriately.
question
190 (Because this calculation involved division, the final answer must be rounded to the same number of significant figures as the measured value with the fewest significant figures.)
answer
Compute 1240.64 / 6.4. Round the answer appropriately.
question
223.402 (Because this calculation involved addition, the final answer must be rounded to the same number of digits to the right of the decimal point as the measured value with the fewest number of digits to the right of the decimal point.)
answer
Compute 214.056 + 9.3456 . Round the answer appropriately.
question
2
answer
How many zeros are significant in the following number? 0.0035060
question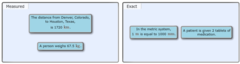

answer
Identify the number in each of the following as measured or exact.
question
The measurement requires a measuring device.
answer
Give the reason for your choice: A person weighs 67.5 kg.
question
The measurement is obtained by counting.
answer
Give the reason for your choice: A patient is given 2 tablets of medication.
question
The statement contains the values in the metric definition.
answer
Give the reason for your choice: In the metric system, 1 m is equal to 1000 mm.
question
The measurement requires a measuring device.
answer
Give the reason for your choice: The distance from Denver, Colorado, to Houston, Texas, is 1720 km.
question
6 oz
answer
Identify the measured number(s), if any, in each of the following pairs of numbers. 3 hamburgers and 6 oz of meat
question
none of the above
answer
Identify the measured number(s), if any, in each of the following pairs of numbers. 1 table and 4 chairs
question
both
answer
Identify the measured number(s), if any, in each of the following pairs of numbers. 0.75 lb of grapes and 350 g of butter
question
none of the above
answer
Identify the measured number(s), if any, in each of the following pairs of numbers. 60 s = 1 min
question
0.0038(0) m
answer
Indicate the significant zeros, if any, in each of the following measurements. 0.00380 m
question
5.(0)4 cm
answer
Indicate the significant zeros, if any, in each of the following measurements. 5.04 cm
question
8(00). L
answer
Indicate the significant zeros, if any, in each of the following measurements. 800. L
question
3.(0)×10?³kg
answer
Indicate the significant zeros, if any, in each of the following measurements. 3.0×10?³kg
question
There are none
answer
Indicate the significant zeros, if any, in each of the following measurements. 85000 g
question
250.0 L
answer
Identify the number that contains more significant figures. 250.0 L 2.5×10?²L
question
0.0120 s
answer
Identify the number that contains more significant figures. 12 000 s 0.0120 s
question
405.0 K
answer
Identify the number that contains more significant figures. 405.0 K 405 K
question
11.00 m
answer
Identify the number that contains more significant figures. 11.0 m 11.00 m
question
4
answer
How many significant figures are in each of the following measurements? 10.09 g
question
2
answer
How many significant figures are in each of the following measurements? 0.000 32 m
question
2
answer
How many significant figures are in each of the following measurements? 36 000 000 m
question
3
answer
How many significant figures are in each of the following measurements? 1.80×10?g
question
4
answer
How many significant figures are in each of the following measurements? 0.8250 L
question
3
answer
How many significant figures are in each of the following measurements? 65.0° C
question
1.70 kg
answer
Round off each of the following measurements to three significant figures. 1.702 kg
question
179 L
answer
Round off each of the following measurements to three significant figures. 178.5238 L
question
4.74×10?³ cm
answer
Round off each of the following measurements to three significant figures. 0.004 738 265 cm
question
7040 m
answer
Round off each of the following measurements to three significant figures. 7042 m
question
1.85×10?? s
answer
Round off each of the following measurements to three significant figures. 1.847×10?? s
question
1.3
answer
Perform each of the following calculations and give answers with the correct number of significant figures: 49.8×0.026
question
1×10?²
answer
Perform each of the following calculations and give answers with the correct number of significant figures: 0.00178×8
question
28.8
answer
Perform each of the following calculations and give answers with the correct number of significant figures: 40.26/1.40
question
2.4
answer
Perform each of the following calculations and give answers with the correct number of significant figures: ((0.2635)(21))/2.32
question
57.16 cm
answer
Perform each of the following calculations, and give answers with the correct number of decimal places: 48.64cm+8.519cm
question
123.9 g
answer
Perform each of the following calculations, and give answers with the correct number of decimal places: 23.55g+100.3g+0.030g
question
125.0 mL
answer
Perform each of the following calculations, and give answers with the correct number of decimal places: 146.905mL?21.0mL
question
0.55 L
answer
Perform each of the following calculations, and give answers with the correct number of decimal places: 1.10L?0.555L
question
5
answer
How many significant figures does each of the following quantities have? 76.600 kJ
question
6
answer
How many significant figures does each of the following quantities have? 4.50200×10³g
question
1,2,3,4
answer
How many significant figures does each of the following quantities have? 3000 nm (Check all that apply)
question
3
answer
How many significant figures does each of the following quantities have? 0.00300 mL
question
This is an exact number
answer
How many significant figures does each of the following quantities have? 18 students
question
1
answer
How many significant figures does each of the following quantities have? 3 ×10??g
question
4
answer
How many significant figures does each of the following quantities have? 47.60 mL
question
3,4
answer
How many significant figures does each of the following quantities have? 2070 mi (check all that apply)
question
24.612
answer
Carry out the following calculations, expressing each result with the correct number of significant figures: 24.567 g + 0.044 78 g = ? g
question
3.65×103
answer
Carry out the following calculations, expressing each result with the correct number of significant figures: 9.2376 g / 0.002 53 L = ? g/L
question
30.1
answer
Carry out the following calculations, expressing each result with the correct number of significant figures: 0.541 mL + 32.1 mL - 2.5833 mL = ? mL
question
3.940 L
answer
Round off each of the following quantities to the number of significant figures indicated in parentheses. 3.940499 L (4)
question
291 K
answer
Round off each of the following quantities to the number of significant figures indicated in parentheses. 291.1514 K (3)
question
91.78 kg
answer
Round off each of the following quantities to the number of significant figures indicated in parentheses. 91.775 kg (4)
question
726.89 kJ
answer
Round off each of the following quantities to the number of significant figures indicated in parentheses. 726.891 kJ (5)
question
T = 32.4° C 3 significant figures

answer
What is the temperature reading on the Celsius thermometer? How many significant figures do you have in your answer?
question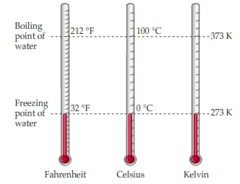

answer
The boiling and freezing points of water are constants. Water boils at 212 °F, 100 °C, or 373.15 K. Although these temperatures are equivalent, the scales used to measure them are different. Water freezes at 32 °F, 0 °C, or 273.15 K. Note that these conversions are based on the fact that water will boil or freeze at a certain temperature regardless of the scale used to measure it.
question
37.0 °C Notice that the conversion factor includes the fraction 5 °C/9 °F. This indicates that for every change of 5 °C, there is a change of 9 °F. Because a change of 1 Celsius degree is approximately the same as a change of 2 Fahrenheit degrees, you can double the temperature in Celsius and add 30 to quickly estimate a temperature in degrees Fahrenheit from a Celsius measurement. This estimation method is useful for Americans traveling abroad.
answer
The average normal human body temperature is 98.6 °F. What is normal body temperature in degrees Celsius?
question
310.2 K
answer
The average normal human body temperature is 98.6 °F. What is normal body temperature in kelvins?
question
?33.3°C
answer
Convert ?28.0 °F to Celsius.
question
T? = 194 K
answer
Carry out the indicated temperature conversions. -79 ?C = ? K
question
T? = 300 °F
answer
Carry out the indicated temperature conversions. 149 ?C = ? ?F
question
T? = 179 °F
answer
Carry out the indicated temperature conversions. 355 K = ? ?F
question
T = 3422 °C
answer
Tungsten, the element used to make filaments in lightbulbs, has a melting point of 6192 °F. Convert this temperature to degrees Celsius.
question
T = 3695 K
answer
Tungsten, the element used to make filaments in lightbulbs, has a melting point of 6192 °F. Convert this temperature to Kelvins.
question
T°F = 325 °F
answer
Suppose that your oven is calibrated in degrees Fahrenheit but a recipe calls for you to bake at 163 °C. What oven setting should you use?
question
2.6 × 10??
answer
Express this number in scientific notation. 0.00026
question
Kilograms
answer
What is the basic SI (Systeme Internationale) unit of mass?
question
kilograms (kg)
answer
Which of the following is a unit of measurement for mass?
question
1340000 = 1.34×10? (So 1.34×106=1.34×10×10×10×10×10×10=1340000.)
answer
Type the number 1340000 in scientific notation.
question
0.000354 = 3.54×10?? So 3.54×10?4=3.54× 1/(10×10×10×10)=0.000354
answer
Type the number 0.000354 in scientific notation.
question
The number 1.23×10? is the greatest number in the group because its exponent is the greatest.

answer
Rank the following numbers.
question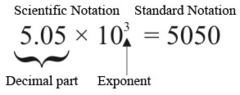

answer
Scientific Notation vs. Standard Notation
question
3.14×10³ = 3140 Thus 3.14×10³=3.14×10×10×10=3140.
answer
Type the number 3.14×10³ in standard notation.
question
8.75×10?? = 8.75×10?? Thus 8.17×10?4=8.17×(1/10x10x10x10)=0.000817
answer
Type the number 8.75×10?? in standard notation.
question
1.27×10??
answer
Convert the number 0.000127 to scientific notation, then enter the answer using the MasteringChemistry format.
question
2.4×10?? The MasteringChemistry format can be used to actually perform the calculations as well as just for entering the final answer. However, be sure that you know how to do the same operations on your calculator when not using MasteringChemistry.
answer
Use your calculator to determine the answer to the following calculation: (1.0×10?¹?/4.2×10??) Express the answer in MasteringChemistry format using two significant figures.
question
1×10?? and 1×10?? Comparison of numbers in scientific notation is sometimes easier to do if all numbers are converted to have the same exponent.
answer
4.3×10?? is between which two numbers?
question
0.5×10¹¹, 50×10?, 500×10? The number 5×10¹? literally means 5 multiplied by 10 to the 10th power, which is equivalent to multiplying 50 by 10 to the 9th power, 500 by 10 to the 8th power, or 0.5 by 10 to the 11th power.
answer
Which of the following are equal to 5×10¹??
question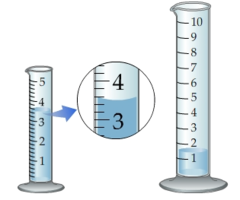
3.54 mL Looking at the "zoomed-in" view, you can see that the bottom of the meniscus is slightly more than halfway between 3.4 and 3.6. The uncertainty in the final digit is actually 0.04 mL to 0.08 mL depending on the quality of the graduated cylinder. The answer is therefore 3.54 ± 0.08 mL. Some may be tempted to round this to 3.5 mL, but that understates the precision in the last digit.

answer
How many milliliters of liquid does the smaller graduated cylinder contain?
question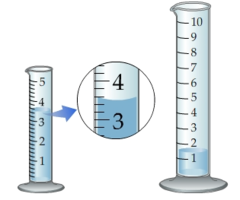
1.5 mL

answer
How many milliliters of liquid does the larger graduated cylinder contain?
question
The smaller.
answer
If you could use only one of these graduated cylinders, which graduated cylinder would more precisely measure a given volume of liquid?
question
volume
answer
I filled my gas tank with 12 L of gasoline.
question
length
answer
My friend is 170 cm tall.
question
length
answer
We are 385000 km away from the Moon.
question
time
answer
The horse won the race by 1.2 s.
question
kilometer length
answer
4.8 km The name of the unit is The type of measurement is
question
milligram mass
answer
325 mg The name of the unit is The type of measurement is
question
milliliter volume
answer
1.5 mL The name of the unit is The type of measurement is
question
seconds time
answer
480 s The name of the unit is The type of measurement is
question
Celsius temperature
answer
28 °C The name of the unit is
question
5.6×10? m
answer
56000 m written in scientific notation?
question
4.3×10² g
answer
430 g written in scientific notation?
question
6×10?? cm
answer
0.000006 cm written in scientific notation?
question
5.1×10?? s
answer
0.00051 s written in scientific notation?
question
4.84×10?³ L
answer
0.00484 L written in scientific notation?
question
6.9×10? kg
answer
690000 kg written in scientific notation?
question
7.2×10³ cm
answer
Which number is larger? 8.2×10²cm 7.2×10³cm
question
3.2×10?² kg
answer
Which number is larger? 4.5×10?? kg 3.2×10?² kg
question
1×10? L
answer
Which number is larger? 1×10?? L 1×10? L
question
6.8×10?² m
answer
Which number is larger? 0.00052 m 6.8×10?² m
question
0.005 m
answer
Write each of the following as standard numbers 5×10?³ m
question
4000000 g
answer
Write each of the following as standard numbers 4×10? g
question
0.0825 kg
answer
Write each of the following as standard numbers 8.25×10?² kg
question
12000 s
answer
Write each of the following as standard numbers 1.2×10? s
question
3.72?10?¹? m
answer
Express the following quantities in scientific notation. The diameter of a sodium atom, 0.000000000372 m.
question
5.58?10? m
answer
Express the following quantities in scientific notation. The distance from the Earth to the Mars 55,800,000 m.
question
Fundamental SI units are base units from which all derived units are formed.
answer
What is the difference between a derived SI unit and a fundamental SI unit?
question
m A kg
answer
Choose the fundamental SI units.
question
Pa mL dm³
answer
Choose the derived SI units.
question
kg m K m³ J kg/m³
answer
What SI units are used for measuring the following quantities? For derived units, express your answers in terms of the six fundamental units. Mass Length Temperature Volume Energy Density
question
1 dm³=1 L
answer
What is the difference between a cubic decimeter (SI) and a liter (metric)?
question
6.02×10¹ km
answer
Which quantity in each of the following pairs is larger? 5.63×10? cm 6.02×10¹ km
question
46 ?s
answer
Which quantity in each of the following pairs is larger? 3.2×10?² ms 46 ?s
question
985,449 g
answer
Which quantity in each of the following pairs is larger? 985,449 g 7.51×10? mg
question
3.6665×10? m³
answer
The Vehicle Assembly Building at the John F. Kennedy Space Center in Cape Canaveral, Florida, is the largest building in the world, with a volume of 3,666,500 m3. Express this volume in scientific notation.
question
7926 mi
answer
The diameter of the Earth at the equator is 7926.381 mi. Round off this quantity to four significant figures
question
7900 mi
answer
The diameter of the Earth at the equator is 7926.381 mi. Round off this quantity to two significant figures
question
270 mg antibiotic/50 kg body mass
answer
Which of the following is a conversion factor for the statement below? A dosage for an antibiotic is 270 mg for 50 kg of body mass.
question
6.71×10? miles/h
answer
The conversion factor relating miles to meters is 1mile=1610m. The speed of light is 3.00×10? m/s. How fast is this in miles per hour (miles/h)?
question
1760 ft²
answer
The conversion factor relating feet to meters is 1 ft=0.305 m. Keep in mind that when using conversion factors, you want to make sure that like units cancel leaving you with the units you need. You have been told that a certain house is 164 m² in area. How much is this in square feet?
question
1947 °F
answer
The melting point of gold is 1064 °C. What is this temperature in degrees Fahrenheit?
question
V = 6×10?¹¹ cm³
answer
How large, in cubic centimeters, is the volume of a red blood cell if the cell has a cylindrical shape with a diameter of 6×10?? m and a height of 2×10?? m?
question
m = 8.88 g m = 0.313 ounces
answer
Gemstones are weighed in carats, with 1 carat = 200 mg (exactly). What is the mass in grams of the Hope Diamond, the world's largest blue diamond at 44.4 carats? What is this mass in ounces?
question
9 pm = 9×10?¹? cm 9 pm = 9×10?³ nm
answer
Carry out the following conversions. 9 pm =____ cm=____ nm
question
8.6 cm³ = 8.6×10?? m³ 8.6 cm³ = 8600 mm³
answer
8.6 cm³ =____ m³=____ mm³
question
62.6 mg = 6.26×10?² g 62.6 mg = 6.26×10¹? pg
answer
62.6 mg =____ g=____ pg
question
m = 110 g
answer
How many grams of meat are in a quarter-pound hamburger (0.25 lb)?
question
h = 443.2 m
answer
How tall in meters is the Sears Tower in Chicago (1454 ft)?
question
A = 7.6181×10¹² m²
answer
How large in square meters is the land area of Australia (2941526 mi2)?
question
5.9 in = 0.15 m
answer
Convert the following quantities into SI units with the correct number of significant figures. 5.9 in .
question
66.53 lb = 30.18 kg
answer
Convert the following quantities into SI units with the correct number of significant figures. 66.53 lb
question
0.5600 gal = 2.120×10?³ m3
answer
Convert the following quantities into SI units with the correct number of significant figures. 0.5600 gal .
question
62 mi/h = 28 m/s
answer
Convert the following quantities into SI units with the correct number of significant figures. 62 mi/h .
question
977.4 yd³ = 747.3 m³
answer
Convert the following quantities into SI units with the correct number of significant figures. 977.4 yd³ .
question
2.390 mi² = 6.190×10? m²
answer
Convert the following quantities into SI units with the correct number of significant figures. 2.390 mi²
question
vcm/shake = 0.57 cm/shake
answer
Among many alternative units that might be considered as a measure of time is the shake rather than the second. Based on the expression "faster than a shake of a lamb's tail," we'll define 1 shake as equal to 2.5×10?4 s. If a car is traveling at 51 mi/h , what is its speed in cm/shake?
question
hcm = 190 cm
answer
The height of a horse is usually measured in hands instead of in feet, where 1 hand equals 1/3 ft (exactly). How tall (in centimeters) is a horse of 18.7 hands ?
question
V = 0.26 m3
answer
The height of a horse is usually measured in hands instead of in feet, where 1 hand equals 1/3 ft (exactly). What is the volume (in cubic meters) of a box measuring 6 × 3.0 × 14 hands ?
question
7.9 g/mL
answer
You add 8.8 g iron to 26.60 mL of water and observe that the volume of iron and water together is 27.72 mL . Calculate the density of iron.
question
12.4 L A 142-kilometer trip is approximately 90 miles so it would make sense that it would take about 8-16 liters (or 2-4 gallons) of gas to make that trip, depending on the efficiency of your car.
answer
In Europe, gasoline efficiency is measured in km/L. If your car's gas mileage is 27.0 mi/gal , how many liters of gasoline would you need to buy to complete a 142-km trip in Europe? Use the following conversions: 1km=0.6214mi and 1gal=3.78L.
question
84.9 dollars After a fairly heavy week of driving, this would be an appropriate amount of money to spend on gas.
answer
While in Europe, if you drive 123 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car's gas mileage is 33.0 mi/gal ? Assume that 1euro=1.26dollars.
question
33.6 km/h Approximately 33-35 kilometers per hour is equal to 20-22 miles per hour. This is the top speed that humans can possibly move on foot. Even the best sprinters in the world would not beat this speed by much. Answers that are much higher than this would not be possible by humans. Answers that are much lower than this would be too slow for a top athlete.
answer
A sprinter set a high school record in track and field, running 200.0 m in 21.4 s . What is the average speed of the sprinter in kilometers per hour?
question
0.560 kg The recommended daily amount of dietary fat (based on a 2000 calories a day diet) is about 67 g (0.067 kg).
answer
A specific brand of gourmet chocolate candy contains 7.00 g of dietary fat in each 22.7-g piece. How many kilograms of dietary fat are in a box containing 4.00 lb of candy?
question
4.43×10? g
answer
A particular brand of gasoline has a density of 0.737 g/mL at 25 ?C. How many grams of this gasoline would fill a 15.9 gal tank (1US gal=3.78L)?
question
7.0 g/cm³ The density of this object is greater than that of water (1.0 g/mL) so it would sink in water. Intuitively, this makes sense as the majority of metals sink in water.
answer
A block of metal has a width of 3.2 cm, a length of 17.1 cm, and height of 3.4 cm . Its mass is 1.3 kg . Calculate the density of the metal.
question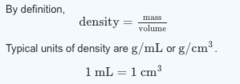
mass = 157 g
answer
The density of iron is 7.87 g/mL. What is the mass of a 5.00 cm × 4.00 cm × 1.00 cm block of iron?
question
V = 4.28 mL
answer
Chloroform, a substance once used as an anesthetic, has a density of 1.483 g/mL at 20 °C.
question
m = 1000 kg
answer
The density of silver is 10.5 g/cm³. What is the mass (in kilograms) of a cube of silver that measures 0.46 m on each side?
question
V? = 0.20 cm³ V? = 1.59×10? cm³
answer
Aspirin has a density of 1.40 g/cm3. What is the volume (in cubic centimeters) of an aspirin tablet weighing 2.8×102 mg ? Of a tablet weighing 492 lb ?
question
? = 11 g/cm
answer
What is the density of lead (in g/cm3 ) if a rectangular bar measuring 0.50 cm in height, 1.55 cm in width, and 25.00 cm in length has a mass of 217.1 g ?
question
2000 mg/L 2000 ?g/mL 2 g/L 2000 ng/?L
answer
Amounts of substances dissolved in solution are often expressed as mass per unit volume. For example, normal human blood has a cholesterol concentration of about 200mg/100mL. Express this concentration in the following units. mg/L ?g/mL g/L ng/?L
question
m = 10 g
answer
How much total blood cholesterol in grams does a person have if the normal blood volume in the body is 5 L?
question
V = 2.52 cm³ N = 4.45×10²³ C atoms
answer
A pure diamond with a mass of 0.1000 g contains 5.014×10²¹ carbon atoms and has a density of 3.52 g/cm³. What is the volume of the Hope Diamond, the world's largest blue diamond at 44.4 carats? (1 carat = 200 mg) How many carbon atoms does it contain?
question
Vft³ = 2.18×10? ft³ V = 3.92×10? acre?feet
answer
The volume of water used for crop irrigation is measured in acre?feet, where 1 acre?foot is the amount of water needed to cover 1 acre of land to a depth of 1 ft. If there are 640 acres per square mile, how many cubic feet of water are in 5 acre?feet? How many acre-feet are in Lake Erie (total volume=116mi3)?
question
Tg,ns,kL Even though all SI prefix-unit combinations are possible, not all of them are commonly used. The prefix centi, for example, is commonly used with meters, but not so commonly with grams, which is why you have probably heard of a centimeter, but not a centigram. The prefix mega, while commonly used with non-SI units such as pixels and bytes, is not often used for meters.
answer
What are the abbreviations for a teragram, a nanosecond, and a kiloliter, respectively?
question

answer
Rank the following quantities in order of decreasing mass.
question
0.01 1000 0.001 10¹² 10? 10?¹²
answer
Write the numerical values for each of the following prefixes. centi kilo milli tera mega pico
question
nanosecond mL cm Mg fL ns
answer
Write the abbreviation for each of the following units: nanometer milliliter centimeter megagram femtoliter nanosecond
question
1 m = 1×10? nm
answer
Complete the following metric relationship.
question
1 dm = 0.1 m
answer
Complete the following metric relationship.
question
1 L = 1000 mL
answer
Complete the following metric relationship.
question
kiloliter
answer
Which is the larger unit? milliliter kiloliter
question
milliliter
answer
Which is the larger unit? milliliter microliter
question
kg
answer
Which is the larger unit? kg g
question
kL
answer
Which is the larger unit? kL dL
question
nanometer
answer
Which is the larger unit? nanometer picometer
question
1 m = 100 cm 1 m/100 cm and 100 cm/1 m
answer
centimeters and meters Conversion factors are
question
1 g = 1000 mg 1 g/1000 mg and 1000 mg/1 g
answer
milligrams and grams Conversion factors are
question
1 L = 1000 mL 1 L/1000 mL and 1000 mL/1 L
answer
liters and milliliters Conversion factors are
question
1 dL = 100 mL 1 dL/100 mL and 100 mL/1 dL
answer
deciliters and milliliters Conversion factors are
question
1 yd = 3 ft 1 yd/3 ft and 3 ft/1 yd
answer
One yard is 3 ft Conversion factors are
question
1 kg = 2.20 lb 1 kg/2.20 lb and 2.20 lb/1 kg
answer
One kilogram is 2.20 lb. Conversion factors are
question
1 min = 60 s 1 min/60 s and 60 s/1 min
answer
One minute is 60 s. Conversion factors are
question
1 gal = 27 mi 1 gal/27 mi and 27 mi/1 gal
answer
A car goes 27 miles on 1 gal of gas. Conversion factors are
question
100 g sterling = 93 g silver 100 g sterling/93 g silver and 93 g silver/100 g sterling
answer
Sterling silver is 93% by mass silver. Conversion factors are
question

answer
Identify the numbers from the statements above as exact or measured.
question
3
answer
Give the number of significant figures for 2.20 lb.
question
2
answer
Give the number of significant figures for 27 miles.
question
2
answer
Give the number of significant figures for 93 g of silver.
question
1 s = 3.5 m 1 s / 3.5 m and 3.5 m / 1 s
answer
Write the equality and give conversion factors for each of the following statements. A bee flies at an average speed of 3.5 m per second.
question
1 day = 3500 mg of potassium 1 day / 3500 mg potassium and 3500 mg potassium / 1 day
answer
The daily requirement for potassium is 3500 mg.
question
1 gal of gasoline = 46.0 km 1 gal gasoline / 46.0 km and 46.0 km / 1 gal gasoline
answer
An automobile traveled 46.0 km on 1 gal of gasoline.
question
1 tablet = 50. mg of Atenolol 1 tablet / 50. mg Atenolol and 50. mg Atenolol / 1 table
answer
The label on a bottle reads 50. mg of Atenolol per tablet.
question
1 kg of plums = 29 ?g of pesticide 1 kg plums / 29 ?g pesticide and 29 ?g pesticide / 1 kg plums
answer
The pesticide level in plums was 29 ppm
question
1 tablet = 81 mg of aspirin 1 tablet / 81 mg aspirin and 81 mg aspirin / 1 tablet
answer
A low-dose aspirin tablet contains 81 mg of aspirin.
question

answer
Identify the numbers from the statements above as exact or measured.
question
2
answer
Give the number of significant figures for 3.5 m.
question
2
answer
Give the number of significant figures for 3500 mg of potassium.
question
3
answer
Give the number of significant figures for 46.0 km.
question
2
answer
Give the number of significant figures for 50. mg of Atenolol.
question
2
answer
Give the number of significant figures for 29 ?g of pesticide.
question
2
answer
Give the number of significant figures for 81 mg of aspirin.
question
1.20 × 10? J
answer
During a daily trip to the gym, you burned 288 kcal by running on the treadmill. Convert this value to joules.
question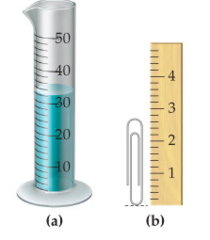
V = 32.1 mL 3

answer
How many milliliters of water does the graduated cylinder in (a) contain? How many significant figures do you have in this answer?
question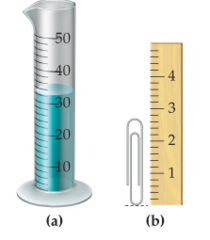
h = 2.78 cm 3

answer
How tall in centimeters is the paper clip in (b)? How many significant figures do you have in this answer?
question
A Celsius degree is larger than a Fahrenheit.
answer
Which is larger, a Fahrenheit degree or a Celsius degree?
question
9/5
answer
By how much?
question
1 cal = 4.184 J 1 ft = 12 in
answer
Which of the following statements use exact numbers?
question
1 ng = 1000 pg
answer
How many picograms are in 1 ng?
question
35 ng = 3.5×10? pg
answer
How many picograms are in 35 ng?
question
5.7×10?? cm
answer
Which quantity in each of the following pairs is smaller? 161 pm or 5.7×10?? cm
question
1.7×10¹¹ ?m
answer
Which quantity in each of the following pairs is smaller? 1.7×10¹¹ ?m or 2.7×10² km
question
3 GA
answer
Which quantity in each of the following pairs is smaller? 3 GA or 3.3×1015?A
question
6 6 4 3 2,3,4,5 5
answer
How many significant figures are in each of the following measurements? 35.0445 g 59.0001 cm 0.03003 kg 0.00450 m 67,000 m² 3.8200 ×10³ L
question
3.568×10? m 3.56801×10? m 71 g 70.7 g 4910 cm 2.3032×10?? kg
answer
Round off the following quantities to the number of significant figures indicated in parentheses. 3.568008 × 10? m (4) 3.568008 × 10? m (6) 70.651 g (2) 70.651 g (3) 4906 cm (3) 2.30323 × 10?? kg (5)
question
10.6 21 4.8×10?² 5527 87.7 14
answer
Express the results of the following calculations with the correct number of significant figures. 4.938 × 2.15 98.03 ÷ 4.7 4.7 ÷ 98.03 5502.5 + 24 + 0.04 86.2 + 1.49 - 0.01 5.7 × 2.53
question
1.93×10? mg/mi
answer
In the U.S., the emissions limit for carbon monoxide in motorcycle engine exhaust is 12.0 g of carbon monoxide per kilometer driven. What is this limit expressed in mg per mile with the correct number of significant figures?
question
? = 2.33 g/cm³
answer
When an irregularly shaped chunk of silicon weighing 8.763 g was placed in a graduated cylinder containing 25.00 mL of water, the water level in the cylinder rose to 28.76 mL. What is the density of silicon in g/cm³?
question
993,1695 °C 1819,3083 °F
answer
Sodium fluoride has a melting point of 1266 K and a boiling point of 1968 K . Convert these temperatures to degrees Celsius. Convert these temperatures to degrees Fahrenheit.
question
V = 71.13 mL
answer
The density of chloroform, a widely used organic solvent, is 1.4832 g/mL at 20 °C. How many milliliters would you use if you wanted 105.5 g of chloroform?
question
200 3.3 mL 26 Cal/kiss 51%
answer
A bag of Hershey's Kisses contains the following information: Serving size: 9pieces= 41grams Calories per serving: 230. Total fat per serving: 13 g The bag contains 2.0 lb of Hershey's Kisses. How many Kisses are in the bag? The density of a Hershey's Kiss is 1.4 g/mL. What is the volume of a single Hershey's Kiss? How many calories are in one Hershey's Kiss? Each gram of fat yields 9 Calories when metabolized. What percent of the calories in Hershey's Kisses are derived from fat?
question
T final = 42.6 °C
answer
A 115 mL sample of water at 300.0 K was heated for 9 min , 28 s so as to give a constant temperature increase of 3.0 °F/min. What is the final temperature of the water in degrees Celsius?
question
126 lb
answer
Weights in England are commonly measured in stones, where 1 stone=14 lb. What is the weight (in pounds) of a person who weighs 9.00 stones ?
question
2300 kJ 6.3 hours
answer
A Big Mac hamburger from McDonald's contains 540 Calories How many kilojoules does a Big Mac contain? For how many hours could the amount of energy in a Big Mac light a 100 watt lightbulb? (1 watt = 1 J/s)
question
In moist air it combines with oxygen to form aluminum oxide, and it reacts with chlorine to form aluminum chloride. It has a mass of 27.3 g, it is hard, it is cold to touch.
answer
Substances have both chemical properties and physical properties. Which of the following properties of aluminum are chemical properties? Which of the following properties of a ice cube are physical properties?
question
Note that two extensive properties such as mass and volume can combine to give an intensive property such as density.
answer
Physical properties can be classified as either intensive properties or extensive properties. An intensive property is independent of the amount of the sample. An extensive property varies with the amount of the sample. Classify the following as intensive or extensive properties of gold.
question
the density, and melting point, of the sample A sample of any substance in the universe could have any given volume. This extensive property does not narrow down the identity of the unknown sample in any way. However, very few substances have a density of 19.3 g/mL, are lustrous yellow in color, and melt at 1064 ?C. For this reason, intensive properties are most useful for identifying unknown substances.
answer
You are given a sample resembling gold. Which of the following properties could be used to help determine whether the sample is really gold?
question
Keep in mind that not all metals are transition metals or inner transition metals. For example, all the elements of groups 1 and 2 are metallic, but they are main group elements.

answer
Classify the following elements as main group elements, transition metals, or inner transition metals.
question
Elements within a group have similar chemical properties.

answer
Classify the following elements as halogens, alkali metals, alkaline earth metals, or noble gases.
question

answer
Match each element to its period.
question

answer
Classify the following elements as metal, nonmetal, or metalloid (semimetal).
question
2,1. This answer illustrates how Dalton's atomic theory explains the law of definite proportions. In any sample of water, no matter how large or small, the ratio of hydrogen atoms to oxygen atoms will always be 2:1 because that is the ratio within one single molecule. Since each ammonia molecule contains three hydrogen atoms, there will always be three times as many H atoms as there are NH? molecules. 9.13×10²? H atoms
answer
What is the ratio of hydrogen atoms (H) to oxygen atoms (O) in 2 L of water? Enter the simplest whole number ratio in order of hydrogen to oxygen, respectively. How many atoms of hydrogen (H) are present in 200 molecules of ammonia (NH?)? A solution of ammonia and water contains 3.50×10²? water molecules and 7.10×10²? ammonia molecules. How many total hydrogen atoms are in this solution?
question
The composition of the atmosphere is determined by a complex interaction of both physical changes and chemical reactions.

answer
Which of following changes that affect the composition of our atmosphere involve physical changes and which involve chemical reactions?
question
condensation of ethanol, and evaporation of ethanol
answer
In a laboratory experiment, a fermenting aqueous solution of glucose and yeast produces carbon dioxide gas and ethanol. The solution was heated by burning natural gas in a Bunsen burner to distill the ethanol that formed in the flask. During the distillation, the ethanol evaporated and then condensed in the receiving flask. The flame of the burner was kept too close to the bottom of the flask and some of the glucose decomposed into a black carbon deposit on the inside of the flask. During this experiment the following changes occurred. Which of these changes involved a physical change and not a chemical change?
question
As early as 400 B.C., Greek philosophers proposed that matter was made up of particles. During the 1800s, John Dalton linked the idea of atoms with the chemical identity of an element. His atomic theory of matter involved the following postulates. 1. Each element is composed of extremely small particles called atoms. 2. All atoms of a given element are identical to one another in mass and other properties, but the atoms of one element are different from the atoms of other elements. 3. Atoms of an element are not changed into atoms of a different element by chemical reactions; atoms are neither created nor destroyed in chemical reactions. 4. Compounds are formed when atoms of more than one element combine; a given compound always has the same relative number and kind of atoms. Scientists later discovered isotopes, which showed that the second postulate was not entirely true, and nuclear reactions, which showed that the third postulate was not true.
answer
Applying Dalton's Atomic Theory
question
N2+O2?2NO In this reaction, the atoms rearrange but they do not change their identities.
answer
Which of the following reactions is possible according to Dalton's atomic theory?
question
6.40 g
answer
The law of conservation of mass states that mass is neither created nor destroyed during a chemical reaction. This can be gleaned from the third postulate in Dalton's series. Magnesium oxide decomposes into magnesium and oxygen. If 16.12 g of magnesium oxide decomposes to form 9.72 g of magnesium, what mass of oxygen gas is also released in the reaction?
question
For two compounds of C and O, the mass ratio of C to O in one compound is a multiple of the mass ratio of C to O in the other compound. The mass ratio of oxygen to carbon in CO is 1.33 for every sample of CO. The mass ratio of oxygen to carbon in CO2 is 2.667 for every sample of CO2. (Elements can combine in different ways to form different substances whose mass ratios are small whole-number multiples of each other.)
answer
When carbon is oxidized in a small amount of oxygen, the principle product formed is carbon monoxide (CO). When the oxidation occurs in a higher concentration of oxygen, the principle product formed is carbon dioxide (CO2). The mass ratio of oxygen to carbon in carbon dioxide (CO2) is twice the mass ratio of oxygen to carbon in carbon monoxide (CO). Identify the statements that are correct.
question
2
answer
Calculate the ratio of the mass ratio of S to O in SO to the mass ratio of S to O in SO2. Estimate that the mass of S = 32 g and the mass of O = 16 g for this experiment. That is, the experimenter has enough sample such that there are 32 g of S in each.
question
In this example you were told that there was enough of element X in each sample such that each had 2 g of X. Atoms actually have masses on the magnitude of 10?²³ g, and chemists talk about the mass of a sample of atoms or molecules in terms of the mass of a mole (6.022×10?²³ molecules) of the species. The units for the atomic mass of an atom are g/mol or amu, reflecting that there are many atoms of the species in the measured sample.
answer
The elements X and Y combine in different ratios to form four different types of compounds: XY, XY?, XY?, and XY?. Consider that there is enough of each sample to contain 2 g of X, and the mass of X is estimated to be 2 g and the mass of Y is estimated to be 4 g. Arrange the following ratios in order of their increasing value.
question
3
answer
Two different compounds are obtained by combining nitrogen with oxygen. The first compound results from combining 46.7 g of N with 53.3 g of O, and the second compound results from combining 22.5 g of N and 77.4 g of O. Calculate the ratio of the mass ratio of O to N in the second compound to the mass ratio of O to N in the first compound.
question
Notice the heavy zigzag line running diagonally across the right part of the table. This line separates metals (below and to the left of the line) from the nonmetals (above and to the right of the line). Metals tend to lose electrons to form positive ions, whereas nonmetals tend to gain electrons to form negative ions.
answer
Metals vs. nonmetals
question
8 The atomic number is the number of protons in one atom of an element. One oxygen atom contains 8 protons. A neutral oxygen atom also contains 8 electrons.
answer
What is the atomic number of the element located in group 16, period 2 of the periodic table?
question
17 Elements in group 17 must gain an electron to have the same number of electrons as the adjacent noble gas. For example, F has 9 electrons, but it would be more stable if it had 10 electrons like Ne. Therefore, F will react to form a 1? ion (with 9 protons and 10 electrons).
answer
If a new element were discovered and it was found to form 1? ions, in which group would you place it in the periodic table?
question
phosphorus
answer
Which of the following elements is not a metal?
question
Water Elements are substances that cannot be broken down any further by chemical means. Water (H?O) is a compound that can be broken down into its elements, hydrogen and oxygen.
answer
Which of the following is not an element?
question
Boron and aluminum
answer
Which of the following elements are found in group 3A or 3?
question
Mg
answer
Which of the following is an alkaline earth metal?
question
When 20.0 g of nitrogen and 32.0 g of oxygen are combined and allowed to react in two separate experiments, both times the product contains 14.0 g of nitrogen and 32.0 g of oxygen.
answer
Which of the following situations demonstrates the law of definite proportions?
question
Atoms of a particular element are alike.
answer
Which of the following statements was formulated by Dalton in his atomic theory?
question
The rusting of the metal is a chemical change.
answer
You happen to be visiting Northern California and you are driving by Suisun Bay, a notorious graveyard for old ships. You notice that all of these ships appear to be rusting away. Which of the following statements is true?
question
Black ice is an example of a physical change.
answer
Black ice is a thin layer of water on a sidewalk or road that has frozen after the temperature has dropped below freezing. It is called black ice because the ice is nearly invisible, especially when driving in a car at night. Which of the following statements is true?
question
The mass of the new mixture will stay the same.
answer
On a balance, you have beakers of AgNO? solution and NaCl solution. When mixed, they will form AgCl(s). What will happen to the mass of the new mixture?
question
FeO
answer
Two iron oxide samples are given to you where one is red and the other is black. You perform a chemical analysis and you find that the red sample has a Fe/O mass ratio of 2.327 and the black has a Fe/O mass ratio of 3.491. You suspect the red sample is simple rust or Fe?O?. What is the chemical formula for the black sample?
question
Hg Sn Ni
answer
Look at the alphabetical list of elements, and find the symbols for the following elements. Mercury (used in batteries) Tin (used in alloys with other metals) Nickel (used in smoke detectors)
question
Cu,Ag,Au

answer
The three so-called "coinage metals" are located near the middle of the periodic table. Use the periodic table to identify them.
question
metal semimetal nonmetal metal metal nonmetal
answer
Identify the following elements as metals, nonmetals, or semimetals. Pt Te Se Sc Mn Ar



