APP 2b – Cancer 1 – Flashcards
Unlock all answers in this set
Unlock answersquestion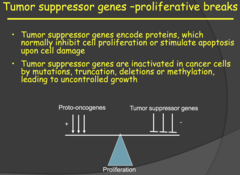
Tumor suppressor genes overview

answer
- Tumor suppressor genes are expressed in pretty much all cells. - Tumor suppressor genes encode proteins, which normally inhibit cell proliferation or stimulate apoptosis upon cell damage - Tumor suppressor genes are inactivated in cancer cells by mutations, truncation, deletions or methylation, leading to uncontrolled growth
question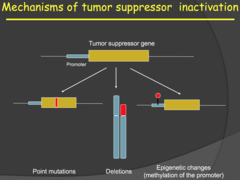
Tumor Supressor Genes - overview (2014)

answer
- Normal cell proliferation is controlled by a balance of Proto-oncogenes and Tumor suppressor genes. - Proto-oncogenes encode for proteins involved in the stimulation of cell proliferation or differentiation. - Tumor suppressor genes encode for proteins which inhibit cell proliferation or stimulate apoptosis. So, activation of the tumor suppressor genes keep our cells from proliferating when there is damage to our DNA - With cancer, the tumor suppressor genes are inactivated or lost. Point mutations, deletions and epigenetic changes frequently result in inactivation of tumor suppressor genes in cancer. - For the epigenetic changes, she specifically mentioned methylation of the promotor sequence for the tumor suppressor gene. This methylation would block transcription of that gene. My notes - It can be caused by deletion or point mutation - It can be an epigenetic change i.e. not a change in genetic sequence but rather a change in methylation of the promoter (no longer accessible to TFs).
question
Hallmarks of cancer - Insensitivity to growth-inhibitory signals (2015)
answer
- If you've ever smoked anything, gotten an x-ray, or been out in the sun, it is likely that your cells were mutated in a fashion conducive to cancer. Our bodies, however are very good at repairing these mutations and stopping these cells from passing on their DNA if they cannot repair the damage. - One method our body uses to fight cancer is through tumor suppressor genes, which inhibit cellular proliferation. These are genes expressed in all of our cells that inhibit cancer development through 3 main mechanisms: 1) Stopping cells from entering the cell cycle before they are ready to divide 2) Repairing DNA that has been damaged 3) Forcing cells to commit programmed cell death if the damage cannot be repaired - Cancer cells must bypass these antioncogenic signals, typically through some sort of random mutation that inactivates the tumor suppressor gene.
question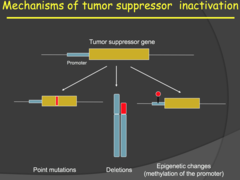
Mechanisms of inactivation (2015)

answer
- Tumor suppressor genes can be inactivated by point mutations, DNA deletions, carcinogens, dimerization, epigenetic changes, or any genetic or transcriptional change that would render a protein useless. - Epigenetic changes involve methylation (silencing) of a promotor sequence in the DNA. - Because we have two alleles of every autosomal gene, we normally need 2 mutations in the same gene for a tumor suppressor gene to lose functionality. This implies that one copy of a tumor suppressor is often enough to suppress oncogenic activity, but there are dosage effects depending on the gene and where/how it is expressed. - This relates to the reason why 1 mutation in oncogene is enough for cancer to grow. It is suspected that tumor suppressors are the first to go, allowing for an increase in cell proliferation signals from oncogenes to hijack cell cycle.
question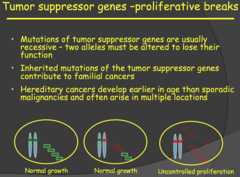
Tumor suppressor genes -proliferative breaks (2015)

answer
- Tumor suppressor gene mutations are usually recessive and we would need inactivation of both alleles for phenotypic effect/uncontrolled proliferation. - This is the Two-Hit Hypothesis. Now, an important note to remember is that inherited mutations of tumor supressor genes contribute to familial cancers. - When you inherit a mutated allele from one of your parents, that mutation is present right at the start (when you are just a zygote). So as you develop, every cell in your body now has one mutated allele (first hit) for that tumor suppressor gene. - You grow and develop normally but have a greater predesposition to cancer because of that mutation. As we know, our DNA is constantly undergoing damage/stress leading to mutations. - Later on in life, you can attain a mutation in the other allele (second hit) for the tumor supressor gene leading to a hereditary cancer. For these reasons, familial/hereditary cancers develop much earlier in life than sporadic cancer (these result if you were two attain both mutations after birth) and can occur at multiple locations in a single individual.
question
Tumor suppressor genes -proliferative breaks (my notes)

answer
- Usually tumor suppressor gene mutations are recessive (unlike oncogenes) i.e. needs both copies to be mutated. It will have some effect with one allele being mutated, but when both are mutated then it is completely altered - The familial inheritance of mutations is usually in tumor suppressor genes rather than oncogenes. This is because if it is tightly regulated, so if oncogenes are mutated, then those children probably die off quick or whatever, while having a recessive tumor suppressor mutation on one allele they are phenotypically normal. This normally manifests in early cancer development and sporadic malignancies
question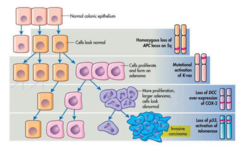
Classical tumor progression (2015)

answer
- In the classical model, tumor development begins with a single cell and the first thing typically is inactivation of a tumor suppressor gene. The tumor is benign at this point. - Mutations will accumulate and proto-oncogenes can become oncogenes and tumor cells can become malignant. Colorectal cancer is prime example of a classical tumor progression. - As you can see on the diagram to the right, you first have a homozygous loss of APC, which as we learned in the first cancer lecture, is a tumor suppressor. This result in a relatively normal looking bunch of tumor cells. - But! Then you have a mutational activation of K-ras (proto-oncogene!) and then a whole cascade of more mutations in tumor suppressors and proto-oncogenes and Boom! You have ugly invasive carcinoma and the colon gets upset. - How does this whole progression cascade thing come about in the first place? Evolution. - Tumor progression results from accumulating random genetic changes and just as the giraffe with the short neck couldn't make it, but the giraffe with the tall neck did, the mutations that allow tumor cells to survive will form tumors. In general, a tumor is a combination of cells with variable mutations and so we call it a heterogenous population.
question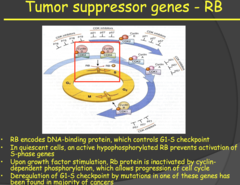
Tumor suppressor genes - RB (2014)

answer
- A very important tumor suppressor gene is the Retinoblastoma (RB) gene which encodes for a DNA-binding protein that controls the G1/S checkpoint of the cell cycle and it is located in EVERY CELL of the body. - Note: many of these genes are named after the diseases that result from their inactivation. - This G1/S checkpoint is the most important checkpoint in the cell cycle. Without control/regulation here, the cells would constantly be proliferating by entering the S phase of the cell cycle. - In a quiescent cell, the RB protein sits at this checkpoint and prevents the transition from G1 to S phase. Through growth factor stimulation of cyclin dependent phosphorylation of the RB protein, the RB protein is inactivated and the cell cycle may progress through S phase. - My notes: To inactivate RB, it must be phosphorylated. If no RB then there is no control stopping the entry into the cell cycle.
question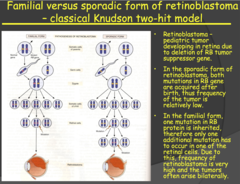
Familial versus sporadic form of retinoblastoma - classical Knudson two-hit model (2014)

answer
- Retinoblastoma is a rapidly developing cancer that develops from the immature cells of the retina and it is the most common malignant tumor of the eye in children but has a low mortality rate. - Important: with SPORADIC Retinoblastoma, one retinal cell received two independent mutations of the RB gene, low probability and low frequency and the cancer is usually unilateral. - With FAMILIAR Retinoblastoma, one mutated allele is carried from birth in all the retinal cells and you only need mutation of the other allele of any of the retinal cells i.e. higher probability and frequency and usually bilateral (both eyes). - Even though every cell of the body carries this gene and can acquire mutations of both alleles, the cancer only develops in the retina, it is very specific. Why is this? - The cells of the body, other than retinal cells, are eliminated by apoptosis when the mutation is acquired in both alleles, quite efficiently when we are young. The developing retinal cells are inefficient at apoptosis and those children that have both mutations preferentially develop Retinoblastoma. - However, later in life, as the cells in our body become less efficient in regulation and apoptosis, tumors can develop elsewhere in the body from these mutations.
question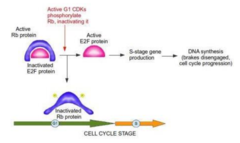
Tumor supressor genes - RB (2015)

answer
a) First discovered in cases of familial retinoblastoma. In these families, offspring inherited 1 defective copy. Being born one step behind leaves these individuals open to a second mutation that would lead to loss of heterozygosity. Keep in mind that, although RB was discovered in families with retinoblastoma and named after the cancer, mutations in the RB tumor suppressor are implicated in MANY cancers, such as osteosarcomas, soft tissue sarcomas, or melanomas. b) Function: Gatekeeper at the G1-S phase checkpoint. This checkpoint is extremely important to prevent the replication of damaged DNA. c) Loss of function: obviously loss of this checkpoint would lead to a green light at the G1- S phase checkpoint at all times. d) If RB is expressed in all cells and guards a very important checkpoint in replication, why do inherited mutations only lead to familial retinoblastomas? Apparently, the only theory is that apoptosis is turned off in retinal cells during development and this increases their dependence on genes like RB, which ensure they replicate with high fidelity.
question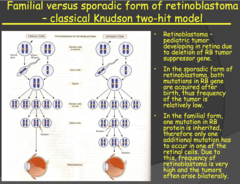
RB (my notes)

answer
Two forms: - Sporadic form: basically both genes occur somatically so is very rare - Familial form which is when there is an inheritance of mutation in RB so every cell in the offspring has only one functional copy of RB so it is much more likely for the good copy to become mutated. - For a person with RB mutation: develops a specific tumor in the eye, why not everywhere else? Because in other tissues is that those affected cells are destroyed/removed but in retina cells there is no elimination of these mutated cells. That is the hypothesis. They can develop other tumors but retina tumors occur first. - Two hit model: postulated that we needed two mutations in tumor suppression genes to have the mutant phenotype
question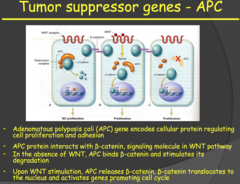
Tumor suppressor genes - APC (2014)

answer
- The APC gene (a tumor suppressor gene) encodes for a protein that interacts with ?- catenin in the WNT pathway. - When the WNT pathway is not activated, the APC protein binds ?- catenin and stimulates its degradation. When the WNT pathway is activated, it promotes the dissociated of the APC protein from ?-catenin. This allows ?-catenin to enter the nucleus and activate genes promoting cell proliferation. - In the absence of the APC gene/protein, ?-catenin is constantly stimulating cell proliferation, which leads to tumor growth. - If you inherit a mutated APC allele, you will develop something called Adenomatous Polyposis coli, polyps in your colon. These are benign and a good warning sign for the development of the malignant familial cancer (with mutation of the second allele), which occurs almost 100% of the time. - You can have many of these polyps in your colon and treatment is to either remove the section containing the polyps or have a complete colonscopy, although the cancer can still develop on the scar tissue.
question
Tumor suppressor genes - APC (2015)

answer
a) Implicated in colon cancer. It begins as benign polyps that progress to cancer nearly 100% of the time by age 40. Even surgical resection of the colon leads to polyps growing in the surgical scar tissue. b) Function: Remember function (1) of tumor suppressor genes. Many tumor suppressors inactivate second messengers of growth factors if the cell is not ready to divide. In this case the growth signal is part of the WNT pathway. When the signaling molecule (WNT) is not present APC inhibits an element of the intracellular signaling cascade (?-Catenin) through ubiquitylation and degradation. c) Loss of function: When APC is not functioning, ?-Catenin will not be degraded (active) and proliferative genes will be permanently turned on. In the absence of an inactivating mutation in APC, an activating mutation in ?-Catenin can lead to the same outcome.
question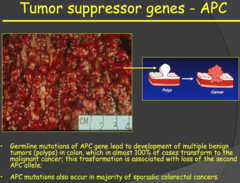
Tumor suppressor genes - APC (my notes)

answer
- Familial colon cancer - Develops as benign tumors due to mutation in APC gene. - APC function binds to B-catenin. If you activate WNT receptor, APC will dissociate from B-catenin and then stimulate TF/proliferation - If you don't have APC gene (two mutations): constitutive activation of B-catenin. = APC patients develop benign tumors/polyps. - Almost 100% chance that the polyps will convert to malignant tumors. - You can remove the whole colon preventatively but often the polyps will grow in the scar tissue.
question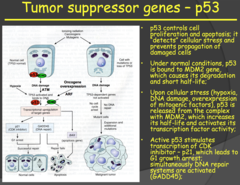
Tumor suppressor genes - p53 (2014)

answer
- p53 is referred to as "The Guardian of the Genome" i.e. very important - In a resting cell, p53 is bound in its inactive state to the MDM2 gene. When the cell experiences stress (hypoxia, DNA damage, oncogene overexpression) p53 is released from MDM2, increasing its half-life and activating its transcription factor activity. - The now released p53 forms a tetramer with other p53 molecules and the now activated form goes on to stimulate transcription of CDK inhibitor - p21, arresting the cell in the G1 phase of the cell cycle, and GADD45, which activates the DNA repair cycle. - If the repair is successful, the cell progresses through the cell cycle. If the DNA repair is unsuccessful, the cell goes through apoptosis. - This tumor suppressor pathway protects us from accumulating abnormal cells and it is present in every cell of the body. - So in summary: p53 (inactivated) bound to MDM2 gene ? cellular stress/damage ? p53 released from MDM2 ? forms a tetramer with other p53 molecules ? p53 tetramer (activated) stimulates transcription of CDK inhibitor - p21 ? cell arrested in G1 ? p53 tetramer stimulates transcription of GADD45 ? DNA repair initiated ? if successful cell continues cycle, if unsuccessful goes through apoptosis
question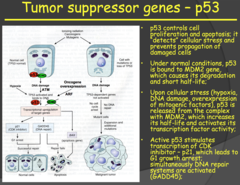
Tumor suppressor genes - p53 (2015)

answer
a) Implicated in many different tumor types. Familial heterozygosity leads to LiFraumeni Syndrome (LFS), which is characterized by early, chronic recurrences of cancer. Fortunately, this inheritance of a mutated p53 gene exhibits incomplete penetrance - not everybody with the mutation develops cancer during their lifetime. b) Function: Initiates caspase cascade of apoptosis i) Normally bound to its "molecular parent," MDM2, but... ii) When there is hypoxia, DNA damage, or oncogene overexpression, p53 is released and allowed to activate TFs that do 2 things (1) Pause the cell cycle and initiate DNA repair mechanisms that we discussed in genetics. If that solves problems, awesome... (2) If not, the cell will undergo apoptosis in order to prevent something bad happening. Something like cancerous cellular proliferation. c) Loss of function: Does not stop cell cycle or kill cell ? DNA damage passed to progeny
question
LiFraumeni Syndrome (LFS) (2015)

answer
- LFS is inherited in a dominant fashion, just like RB. The reason: p53 forms a tetramer that acts like a TF to bind DNA. - Mutations in p53 usually occur in the DNA binding domain, so they affect where p53 binds and what gets transcribed. - There are 3 types of mutations p53 typically undergoes and 2 out of 3 the mutations can lead to cancer in a dominant fashion (without a mutation in the second p53 gene). i) Loss of function - similar to loss of function of the two above. Individual is still protected until loss of heterozygosity - until the second allele is mutated. ii) Dominant negative mutation - because p53 works as a tetramer the mutated proteins that are still transcribed may bind to the normal p53 proteins and cause them to lose function too. I think of this as similar to how prions can cause normal proteins in the brain to misfold. iii) Gain of function - p53 can mutate to bind to a different DNA segment that stimulates proliferative genes, effectively making the mutated p53 an oncogene. As discussed in the previous lecture, this is common in translocation mutations. iv) *P53 doesn't act alone, so any mutation in the pathway may supplement its effect as an oncogene
question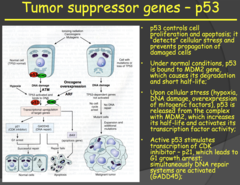
p53 (my notes)

answer
- Very important: p53 - Almost every tumor type you will have alterations in p53 (guardian of the genome) - P53 normally bind to MDM2. - If something is wrong with the cell, the p53 will detach from MDM2 and will do two things: 1. it will stop the cell cycle and initiate DNA repair. If it is not successful in repairing DNA, it will initiate apoptosis - If you don't have functional p53: allows cells that have DNA damage to survive and proliferate. - P53 activation occurs through hypoxia, DNA damage, and oncogene overexpression (MYC, RAS). - Since oncogenes activate p53, it means that in tumors/cancer growth is dependent on tumor suppressor gene inactivation occurring first.
question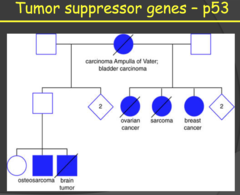
p53 diagram

answer
- Family from LFS of previous slide - Pretty much every kind of type of tumors, which is what you expect with such a universal gene as p53. - We do NOT inherit cancer. What we inherit is susceptibility for cancer. - When you look at the number of family members. It seems dominant which is not what we would expect. Why is that? See next slide
question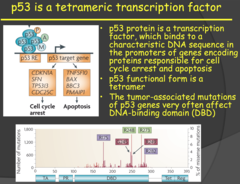
Importance of these tumor suppressor pathways and LFS (2014)

answer
- In the presence of functioning and active tumor suppressor, oncogenes can't accumulate and form cancers. - The protection from these pathways kills or repairs the cell before the mutations can accumulate and form a cancer. - Therefore, in general, the theory of cancer progression is that we lose tumor suppressor protection and then we can generate oncogene mutations. - Germline mutations, those that are inherited by the offspring, in the p53 gene cause LiFraumeni syndrome (LFS). - This is associated with an elevated risk of developing a number of tumors including soft-tissue sarcomas, osteosarcomas, brain tumors, and breast cancer. - Like other familial tumors we have discussed, these develop at an earlier age than sporadic tumors and are seen in multiple locations in an individual (because the germline mutation is present in all cells of the offspring's body). - This germline p53 mutation is an exception to the general rule that tumor suppressor gene mutations are recessive, this one is dominant. This dominant characteristic of LFS is due to the way p53 functions (DNA binding and tetramer activation).
question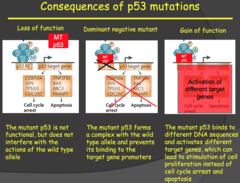
p53 Continued (2014)

answer
- In a simple loss of function mutation, the p53 proteins encoded by the mutated allele lose their function but do not interfere with the other functional p53 molecules that are forming tetramers and binding to DNA. - Therefore, the loss of function mutation is recessive. If a loss of heterozygosity does occur (mutation now in both alleles), it will lead to LFS. - In the dominant negative mutation, a mutant p53 encoded for by the mutant allele binds to the other functioning wild type p53 molecules in the tetramer and prevents the tetramer from functioning. - In this case you only need one mutated allele for the phenotype to result, therefore it is a dominant mutation (as the name implies). The gain of function mutation is even worse. - Once again we have only one mutated allele giving rise to the mutant p53 which binds to the other wild type p53 molecules forming a tetramer that doesn't bind the p53 binding domain on DNA. - However, this tetramer can bind a completely different DNA binding domain and stimulate transcription there. This could be the binding domain for genes involved in cell proliferation. Dr. Kitlinska wrapped up LFS by mentioning that mutations in the proteins anywhere along the p53 pathway can contribute to LFS.
question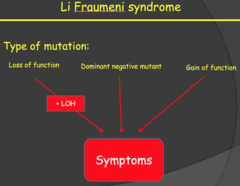
Li Fraumeni syndrome (my notes)

answer
Loss of function is best case because it still acts as a recessive gene, but the other two act as dominant mutations
question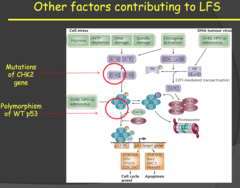
Other factors contributing to LFS (my notes)

answer
Doesn't necessarily have to be p53, it can be something in the pathway that will cause similar symptoms
question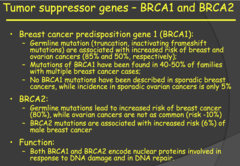
BRCA1 and BRCA2 (Breast Cancer Predisposition Gene 1 and 2) (2014)

answer
- These two tumor suppressor genes encode proteins involved in response to DNA damage and DNA repair. - Sporadic cancers are rarely seen with these two genes. Two key statistical differences are highlighted on slide 47 for the germline mutations of these genes. - BRCA1 germline mutations are associated with 85% increased risk of familial breast cancer and 50% increased risk of familial ovarian cancer. - BRCA2 germline mutations are associated with 80% increased risk of familial breast cancer but only 10% increased risk of familial ovarian cancer. - Also, BRCA2 germline mutations are associated with a 6% increased risk of male breast cancer.
question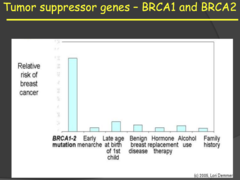
Tumor suppressor genes - BRCA1 and BRCA2 (my notes)

answer
- Mutations in these two genes have the greatest contribution, by far, to increased risk of developing breast cancer. - My notes: Risk is highest with BRCA1-2 for breast cancer but it is somehow low for sporadic tumors? 2015 MNTS: a) Implicated in increased risk of breast and ovarian cancer (85% ; 50%, respectively) in females and an increased risk of male breast cancer (6%) i) BRCA 1/2 mutations increase probability of breast cancer more than all other risk factors. ii) This is why many women (like Angeline Jolie) opt for elective double mastectomies or removal of their ovaries after they no longer plan to have children or breast feed. b) Function: encode proteins involved in DNA damage/repair
question
Tumor suppressor genes - DNA repair genes (my notes)

answer
- Why are DNA mutations important? - What does DNA damage do? It causes mutations. How do we avoid affect of DNA damage? We repair it. If we have mutations in suprressor genes, then those repair mechanisms don't function. - HNPCC: associated with defects of DNA mismatch repair genes (problems with DNA repair) and they basically skip the benign stage of tumors and have malignant tumors immediately. - Xeroderma pigmentosum: you induce DNA damage. Very sensitive to light and basically cannot go outside because they cannot repair DNA damage.
question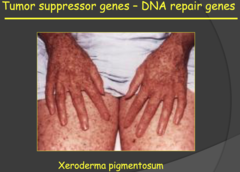
DNA Repair (2014)

answer
- Dr. Kitlinska said that these are not tumor suppressor genes per say but are included in the same category as tumor suppressor genes because they maintain other tumor suppressor genes through their function and loss of these DNA repair genes can lead to the accumulation of oncogenes and the loss of the protective mechanisms of tumor suppressor genes. - Thus, mutations of DNA repair genes can lead to cancer, just like with mutation in tumor suppressor genes. - Hereditary nonpolyposis colorectal cancer (HNPCC) was one of two examples of disease associated with mutations of DNA repair genes. This cancer is associated with defects in the DNA mismatch repair gene and, unlike APC, there are no polyps in the colon to serve as warning signs for the development of this cancer. - Xeroderma pigmentosum was the other disease mentioned. The phenotypes of this condition, lesions on skin, result from defects of the nucleotide excision repair system that is responsible for repair of DNA damaged from UV-light every time we walk out into the sun. Individuals with this condition are at a greater risk of developing UV-induced skin cancer.
question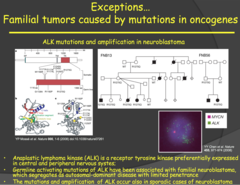
Exceptions... Familial tumors caused by mutations in oncogenes (my notes)

answer
- Neuroblastoma has a familial form: Scientists couldn't find a tumor suppressor genes for it. They found it was because of an oncogene. ALK receptor is expressed preferentially in the CNS. This is why fetus can appear normal because it is specific to certain tissues. - Mutation itself is not enough (it appears recessive). There are other factors such as environmental and otherwise that lead to these tumors. - In the image we see there is only MYCN but multiple copies of ALK
question
Examples of defects in DNA Repair Genes: Hereditary Nonpolyposis Colorectal Cancer ; Xeroderma Pigmentosum (2015)

answer
- Nonfunctional DNA repair genes (function 2 of tumor suppressors) can lead to cancer. This makes sense. Although not directly involved in cell cycle regulation, DNA repair genes are necessary to prevent potentially deleterious mutations from being passed down to other cells. 1) Hereditary Nonpolyposis Colorectal Cancer: This type of colorectal cancer is similar to APC, but, as the name suggests, polyps that warn of future malignancies do no develop. - Associated with a defect in genes that code for mismatch repair (MMR) proteins 2) Xeroderma Pigmentosum: Defect in UV crosslinking (dimerization) repair proteins - This happens to our exposed skin all the time, so defects in this repair process illustrate how dependent we are at all times on our body stepping up to protect us
question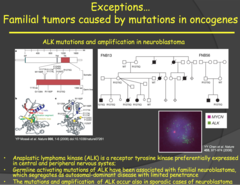
Anablastic Lymphoma Kinase (ALK) (2014)

answer
- The ALK gene is a proto-oncogene that encodes for a receptor tyrosine kinase that is expressed preferentially in central and peripheral nervous systems. - Germline mutations of this gene have been associated with familial neuroblastoma. - NOTE: this is an exception to the rule mentioned earlier that mutations of tumor suppressor genes are responsible for familial cancers. You wouldn't expect an oncogene to results in familial cancer because an oncogene is a dominant mutation and it should prevent a fetus from developing properly if inherited. - In this case, a fetus that inherited this mutation can survive to develop the neuroblastoma because the ALK gene is specific to neuronal tissue (CNS and PNS) and is expressed later in the development of the fetus during the final stages of neuronal development. - Takehome point: inheriting the ALK mutation alone does not lead to development of neuroblastoma and, even if the cancer develops, there is a variety in its aggressiveness. Therefore, although the mutation is dominant, there needs to be other factors (which we yet don't know) involved for the development of the cancer. This could be another reason why children with this oncogene survive development.
question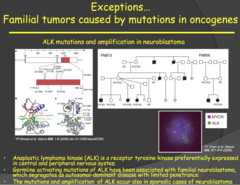
Familial tumors caused by mutations in oncogenes - ALK (Anablastic Lymphoma Kinase) (2015)

answer
- Associated with familial neuroblastomas, lymphomas, and adenocarcinomas of the lung. Plays an important role in development of the brain and is expressed mainly in neurons. - Because nerve cells do not proliferate like other cells of the body, especially in utero, babies are able to survive without uncontrolled proliferation terminating the pregnancy - Function: Tyrosine Kinase receptor - Loss of function: Usually in receptor domain; can lead to ALK being constitutively active or fused to another protein - Not 100% penetrance - suggests environmental factors at play
question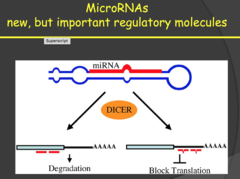
How do MicroRNAs prevent cancer?

answer
2014: - MicroRNAs don't encode for anything but bind to mRNA and block translation or degrade the mRNA. Therefore, these are regulatory molecules that prevent protein expression. - MicroRNAs can also be oncogenic or tumor suppressors depending on their target. Oncogenic MicroRNAs target and eliminate tumor suppressor genes and tumor suppressor MicroRNAs target and eliminate oncogenes. - Disruption of this balance can result in the development of malignant tumors. - My notes: miRNAs: small pieces of RNA that are non coding that regulate other gene expression. They can either degrade or block translation. siRNA is the synthetic version of miRNA (endogenous).
question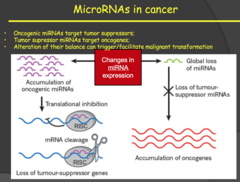
miRNAs (2015)

answer
- microRNAs are small segments of RNA that bind to a complementary sequence of RNA and either block translation or target the sequence to be degraded in a RISC complex. - This means microRNAs can respond quickly to potential oncogenes because they can be transcribed more quickly that larger tumor suppressor genes. - We are just now discovering that they can have just as much impact as tumor suppressor genes in the body's battle against cancer. Therefore, loss of antioncogenic microRNAs is similar in scope to loss of tumor suppressor genes - My notes: miRNAs: some of them will bind to tumor suppressors or to the oncogenes, changing their expression. Basically loss of miRNAs for oncogenes or increased miRNAs on tumor suppressors
question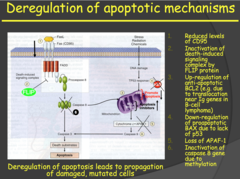
Evasion of Apoptosis (2014)

answer
- Dr. Kitlinska said not to try and memorize all the details of slide 55, but you know, we've heard that a lot before. - Apoptosis has both internal and external pathways. On the slide is listed a number of ways these pathways can be deregulated leading to the uncontrolled proliferation of the cancer cells. - The internal apoptosis pathway is activated by DNA damage/stress (p53 is part of this internal apoptosis pathway). -The external pathway is activated by apoptotic factors from the environment/other cells bind to receptors on the cell and trigger the external apoptotic pathway. - An important molecule in this external pathway is Caspase 8. This molecule is involved in cell death associated with detachment from other cells (think epithelial cells, they have to be attached together to survive). - When this molecule is missing or has lost its function, the cell won't go through apoptosis when it detaches from the other cells. This is how epithelial cancer cells metastasize to other locations.
question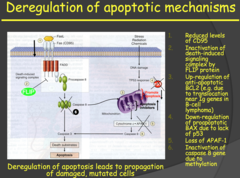
Hallmarks of cancer - Deregulation of apoptotic mechanisms (2015)

answer
- Avoiding apoptosis - typical destiny of mutated cells discovered by tumor suppressors or T-Cells - Two apoptotic pathways: Intrinsic, extrinsic - Deregulation of apoptosis (like in p53) leads to propagation of damaged or mutated cells My notes: - Just know that a deregulation of apoptotic mechanisms can occur in many places. - It can be external signals or internal signals to initiate apoptosis. - In pretty much every step you can have problems. One of the major problems is not having p53
question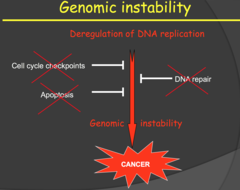
Hallmarks of cancer - Genomic instability (2015)

answer
- Our DNA is tightly regulated, so obviously damage causes huge problems - Refer back to karyotype - Not any progeny of a cancer cell will have same genetics. There is no way to ensure the DNA is evenly split - cells are replicating to fast, centromeres are lost, etc.
question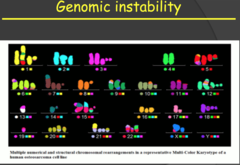
Genomic instability (my notes)

answer
Karyotype of osteocarcinoma. Notice that there are multiple copies and a lot of translocations. Huge amount of genomic instability, progeny of these cells will probably be even more unstable.
question
Limitless Replicative Potential (2014)

answer
- Telomeres are repetitive nucleotide sequences at the end of chromatids that function to protect important cellular DNA from damage/loss during the replication process. - With each replication these protective caps are shortened until a critical length is reached where the telomeres can no longer protect the DNA and the cell seizes to replicate. - Because replication is the means by which cells replenish themselves, after the telomeres are lost we see cellular aging (nonreplicative senescence) and eventually apoptosis. - Some cells, like stem cells, have the ability to restore telomere length using an enzyme called telomerase, keeping the cells "young" and proliferating. Some tumor cells, not all, also activate telomerase allowing them to proliferate for longer cycles. - However, these tumor cells, with the activated telomerase, don't have long telomeres. The cancer cells proliferate at such a high rate that they actually have short telomeres but the telomerase is keeping the telomere length adequate enough to allow for unlimited cell division and to prevent senescence or apoptosis.
question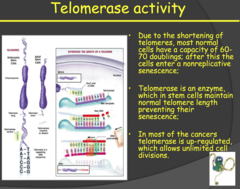
Limitless Replicative Potential (2015)

answer
- Telomerase extends telomere repeats. Recall that at the end of the DNA sequence the polymerase can't quite replicate to end. Telomerase adds more hexamer repeats to enable more replicative cycles in cells that need to divide a lot (like stem cells). - Cancer is continuously replicating, so a telomerase mutation that allows continuous activity is necessary to escape cellular senescence. - Otherwise, the cancer would be self-limiting after a few dozen replications. o Telomeres are still short in cancer cells because the cells replicate so fast that telomerase is just doing enough to keep up with replication My notes: - Limitless replicative potential: at the end of DNA sequences we have telomeres. Lose pieces of telomeres with every replication. - Normal cells that can proliferate for a long time in our bodies but is normal: stem cells. This occurs because they have telomerase that elongates the ends of the chromosome after each division



