Organic Chemistry GCSE – Flashcards
Unlock all answers in this set
Unlock answersquestion
What is organic chemistry?
answer
The study of families of carbon compounds where carbon atoms are found bonded together to form chains and/or rings. (It does not include carbon dioxide, carbon monoxide or carbonate compounds.)
question
What is a homologous series?
answer
A homologous series is a family of compounds. The family members: - 1. share the same general formula. 2. have similar chemical properties. 3. show a gradation in physical properties. 4. differ by a CH₂ group.
question
Explain what the term hydrocarbon means.
answer
A molecule made up of hydrogen and carbon ONLY.
question
Explain what the term saturated means
answer
A substance made of molecules that do not have double or triple bonds between carbon atoms.
question
Explain what the term unsaturated means
answer
Refers to molecules that have at least one double C=C bond (or triple bond).
question
What does the term molecular formula mean?
answer
Formula giving the actual number of atoms of each element in a molecule. e.g. C₄H₁₀ for butane
question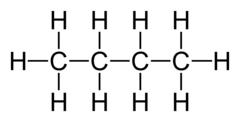
What does the term structural formula mean?

answer
Formula showing how the atoms are bonded together in a molecule. Butane shown here.
question
What does the term empirical formula mean?
answer
Formula that gives the lowest whole-number ratio of atoms present in a molecule. For butane: C₂H₅
question
What does the term functional group mean?
answer
A group of atoms responsible for the characteristic reactions of a particular compound. C=C for alkenes OH for alcohols COOH for carboxylic acids Alkanes don't have a functional group and are the least reactive homologous series.
question
What is the simplest homologous series? The alkanes or alkenes
answer
The alkanes. Alkanes do not have a 'functional group' and are the least reactive homologous series.
question
Are the alkanes saturated or unsaturated hydrocarbons?
answer
The alkanes are saturated hydrocarbons. Propane shown here.
question
Name the first six alkanes
answer
Methane, ethane, propane, butane, pentane and hexane. (Then heptane, octane, nonane and decane.)
question
Write the general formula for alkanes
answer
CnH2n+2 The n and 2n+2 should be subscripts. 2n indicates that for every carbon in the chain there are 2 hydrogens. The +2 indicates the need for a further 2 hydrogens to complete the two ends of the chain.
question
An alkane has 16 Carbon atoms, what is its molecular formula
answer
C₁₆H₃₄
question
What is our main source of alkanes?
answer
Fossil fuels.
question
How are alkanes separated from the mixture of hydrocarbons in crude oil?
answer
By Fractional Distillation.
question
Give a brief outline of fractional distillation (fractionation) of crude oil.
answer
Oil is heated to 360°C. All but the bitumen fraction vaporize. The vapour condenses as it ascends the fractionating column. The higher the b.p. the sooner it condenses. The refinery gases (methane - butane) leave at the top.
question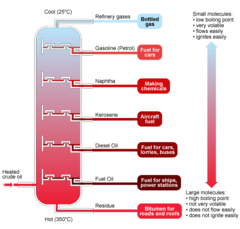
A labeled diagram of a fractionating column.

answer
(Top - Down): Refinery gases Petrol Naphtha Paraffin Diesel Fuel oil Bitumen
question
How does the viscosity (runniness) of the crude oil fraction change as you move from petrol to fuel oil
answer
Viscosity increases. The fractions become less runny.
question
How does burning of the crude oil fraction change as you move from petrol to fuel oil
answer
They burn less and less well and produce increasing amounts of black smoke and soot (unburnt carbon). From paraffin-down, a wick need to be used to keep the fraction burning.
question
Why do alkanes require a lot of energy to break their bonds?
answer
Because the C-C and C-H covalent bonds are strong, requiring a relatively large amount of energy to break them apart.
question
Are the alkenes saturated or unsaturated hydrocarbons?
answer
Alkenes are unsaturated hydrocarbons. All members of the alkene family have one C=C bond.
question
What is the general formula for alkenes?
answer
CnH2n The n and 2n should be written as subscripts. They begin at n=2 (ethene) as you need at least 2 carbons to have a C=C in the molecule.
question
What makes alkenes more reactive than alkanes?
answer
Alkenes have a C=C functional group. One of the covalent bonds of the double bond is weaker than the other, making it more reactive.
question
What happens when bromine water is added to an alkene?

answer
The orange coloured bromine water is immediately decolourised. The image shows a liquid alkene mixed with bromine water. The liquids are immiscible, but when shaken together the orange colour is decolourised.
question
What type of reaction occurs when alkenes react with bromine?
answer
An ADDITION REACTION C₂H₄ + Br₂ → C₂H₄Br₂ 'addition' as 2 molecules add together to give a single molecule.
question
What can be used to distinguish between an alkane and an alkene?
answer
The test with bromine water. With the alkane the bromine water stays orange, but with the alkene it is decolourised.
question
How is crude oil formed?
answer
Formed over millions of years when plankton and other sea creatures die, fall to the sea bed and become covered by layers of sand and silt. Sand and silt means that no oxygen gets to the dead bodies. The heat and pressure builds up and slowly turns them to hydrocarbons.
question
What are the conditions like at the top of the fractionating column? Describe the molecules you would find there.
answer
Low temperatures, about 70 degrees. You would find smaller molecules which are light in colour, very volatile, have a low viscosity and are very flammable.
question
What are the conditions like at the bottom of the fractionating column? Describe the molecules you would find there.
answer
High temperatures, about 360 degrees. You would find larger molecules which are dark in colour, difficult to light, have a high boiling point, low volatility and thick and viscous.
question
What does the fractional distillation of crude oil do to the crude oil?
answer
It separates the mixture of hydrocarbons into more useful products.
question
The fractional distillation of crude oil is described as a continuous process. What does this mean?
answer
Constantly feeding in vapourised crude oil and constantly removing fractions as they are formed. The opposite is a 'batch process' such as is used in the brewing industry.
question
What is a fuel
answer
A fuel is a substance that burns to release energy.
question
What is a fraction?
answer
A fraction is a group of similar sized molecules with similar boiling points.
question
What is a carbon chain?
answer
A line of connected carbon atoms that have covalent bonds.
question
If alkanes burn in a plentiful supply of oxygen what do they produce?
answer
Carbon dioxide and water. This is called 'complete combustion'.
question
Complete and balance the following 2 equation for complete combustion: CH₄ + O₂ → C₄H₁₀ + O₂ →
answer
CH₄ + 2O₂ → CO₂ + 2H₂O C₄H₁₀ + 6¹/₂O₂ → 4CO₂ + 5H₂O or 2C₄H₁₀ + 13O₂ → 8CO₂ + 10H₂O
question
What is incomplete combustion in reference to hydrocarbons and what are the products.
answer
Incomplete combustion happens when the hydrocarbon is burnt in a poor supply of oxygen. Carbon monoxide and steam are the products. CO is known as the 'silent killer'/
question
Does incomplete combustion produce as much energy as complete combustion.
answer
No. It produces significantly less energy. e.g. for methane: complete (890 units of energy released) incomplete (283 units of energy released)
question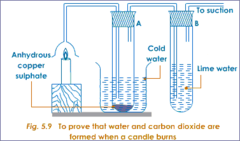
In the experiment to test the combustion products of methane what was the purpose of the inverted funnel?

answer
To increase the surface area in order to catch as much combustion products (gas) as possible.
question
In the experiment to test the combustion products of methane what was the purpose of the ice/water mixture?
answer
To condense the water vapour to water.
question
In the experiment to test the combustion products of methane what was the purpose of the vacuum pump?
answer
To draw (suck) the combustion products through the apparatus.
question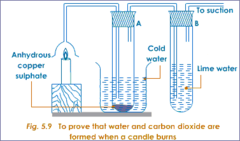
In the experiment to test the combustion products of methane what was the purpose of the anhydrous copper (II) sulfate and limewater.

answer
White anhydrous copper sulfate turns from white to blue when water is added. Limewater turns from clear and colourless to cloudy/milky when CO₂ is added.
question
Write down any observations that you would see in the experiment to test the combustion products of methane.
answer
Lime water turns cloudy showing carbon dioxide is present. Anhydrous cobalt chloride paper turns from blue to pink showing water was present. Anhydrous copper sulphate turned from white to blue showing water was present.
question
Write down a conclusion you could take from the experiment to test the combustion products of methane
answer
Combustion products of methane are carbon dioxide and water (in a plentiful air supply)
question
What happens if a hydrocarbon fuel is burnt in an insufficient oxygen supply?
answer
Incomplete combustion occurs.
question
What are the products of incomplete combustion?
answer
Carbon monoxide and carbon.
question
Describe the characteristics of carbon monoxide and the harmful biological consequences of inhaling it.
answer
Carbon monoxide it a tasteless, colourless, odourless gas that combines with haemoglobin in the red blood cells. The blood cannot carry as much oxygen. It causes drowsiness and headaches and can eventually lead to death.
question
Describe the characteristics of soot (unburnt carbon) and the biological consequences of inhaling it.
answer
Carbon is a black powder. It is seen as soot. Inhaling carbon particulates worsens asthma and other breathing problems.
question
Ethanol is a member of which homologous series?
answer
The alcohols. Proper name alkANOLS. e.g. ethANOL
question
What is the functional group of all alcohols?
answer
An -O-H group, known as a hydroxyl group.
question
What is the general formula for ethanol?
answer
CnH2n+1OH The n and 2n+1 should be subscripts.
question
What are the two ways in which ethanol can be manufactured?
answer
1. Fermentation of sugars 2. Hydration of ethene
question
What materials and conditions are required for fermentation?
answer
Materials: Sugar and Yeast. Conditions: Warmth (35-40⁰C) Anaerobic (oxygen kept out)
question
What would result if oxygen was present during fermentation?
answer
Ethanol would form, but it would then oxidise to ethanoic acid (vinegar).
question
What is the use of lime water in fermentation of sugars in order to make ethanol?
answer
1. Test for whether CO2 is released 2. lets out CO2 but prevents air from entering the yeast - acts as an airlock 3. Shows the rate of fermentation
question
What is the boiling point of ethanol?
answer
78⁰C
question
What determines the alcoholic drink? Give an example
answer
The source of sugar. Wine from grapes. Beer from hops. Cider from apples.
question
How is ethanol manufactured in the hydration of ethene method?
answer
Adding steam to ethene.
question
Write a balanced symbol equation, including state symbols, for the hydration of ethane.
answer
C₂H₄(g) + H₂O(g) → C₂H₅OH(g) Hydration means to react with water/steam. This is also classified as an 'addition reaction'.
question
What conditions are needed for the manufacture of ethene in the hydration of ethene method?
answer
Temperature of 300 ⁰C. Pressure of about 60-70 atm. Catalyst of phosphoric acid.
question
What type of reaction occurs during the hydration of ethene method to make ethanol?
answer
Addition reaction. C₂H₄(g) + H₂O(g) → C₂H₅OH(g) 2 molecules combine to form just 1.
question
Which process to make ethanol is continuous?
answer
Hydration of ethene. The product is removed at one end and more reactants passed in at the other. The 'batch process' used in brewing/fermentation, is less efficient, but it doesn't require the same high temperatures and pressures to be maintained.
question
Is sugar cane a renewable source of energy?
answer
Yes. In some countries several harvests of sugar cane are possible in the same year.
question
Is crude oil a renewable source of energy?
answer
No, it is finite. To be renewable it need to be replaceable within a lifetime. Crude oil took many millions of years to form.
question
Complete and balance the following equation to show the complete combustion of ethanol: C₂H₅OH + O₂ →
answer
C₂H₅OH +3O₂ → 2CO₂ + 3H₂O
question
Complete and balance the following equation to show the incomplete combustion of ethanol: C₂H₅OH + O₂ →
answer
C₂H₅OH + 2O₂ → 2CO + 3H₂O
question
What is methylated spirits?
answer
Methylated spirits is 99% ethanol. The rest is poisonous methanol and tiny amounts of chemicals intentionally added to deter drinking.
question
Give several uses of ethanol.
answer
Alcoholic beverages. Solvent for cosmetics. A fuel.
question
What is the general formula of a carboxylic acid? Proper name alkANOIC ACIDS. e.g. ethANOIC ACID
answer
CnH2n+1COOH The n and 2n+1 should be written as subscripts. In assigning the prefix of the name (e.g. eth) you must count the C atom of the COOH functional group.
question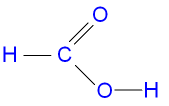
The name and molecular formula of the first member of the carboxylic acids. [Hint: in this case use n = 0)

answer
Methanoic acid HCOOH
question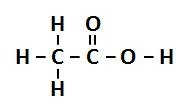
For ethanoic acid, give both its molecular and structural formulae.
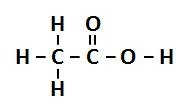
answer
molecular: CH₃COOH structural: shown in image
question
A 5% solution of ethanoic acid in water is better known as
answer
vinegar
question
Describe a test to distinguish between an alcohol and a liquid alkane.
answer
Heat with acidified potassium dichromate in a test tube placed in a boiling water bath. With the alkane the orange colour will remain, but with the alcohol, it will change to dark green.
question
The pH of a dilute solution of ethanoic acid.
answer
3
question
Complete and balance the following equation to show how ethanoic acid reacts with magnesium: Mg + CH₃COOH → Name the organic salt formed.
answer
Mg + 2CH₃COOH → Mg(CH₃COO)₂ + H₂↑ This is a 'MASH' reaction. Magnesium ethanoate is the organic salt.
question
Complete and balance the following equation to show how ethanoic acid reacts with sodium hydroxide solution: NaOH + CH₃COOH → Name the organic salt formed.
answer
NaOH + CH₃COOH → CH₃COONa + H₂O This is a 'neutralisation' reaction. Sodium ethanoate is the organic salt.
question
Complete and balance the following equation to show how ethanoic acid reacts with sodium carbonate solution: Na₂CO₃ + CH₃COOH → Name the organic salt formed.
answer
Na₂CO₃ + 2CH₃COOH → 2CH₃COONa + H₂O + CO₂↑ Sodium ethanoate is the organic salt.
question
Give 2 uses of ethanoic acid.
answer
Vinegar. Pickling. Limescale remover.
question
Complete the following equation to show the polymerization of ethane. n H₂C=CH₂ →
answer
n H₂C=CH₂ → (H₂C-CH₂)n The image shows it better. n = several thousand
question
Give several uses of polythene.
answer
Plastic shopping bags. Plastic toys. Plastic sheets. Plastic aprons and gloves.
question
Complete the following equation to show the polymerization of chloroethene. n H₂C=CHCl →
answer
n H₂C=CHCl → (H₂C-CHCl)n The image shows it better. n = several thousand. chloroethene used to be called vinylchloride
question
Give several uses of polychloroethene (polyvinylchloride PVC)..
answer
Plastic windows & guttering. Vinyl flooring. Waterproof clothing.
question
What kind of polymers are polythene and PVC?
answer
Addition polymers. The other type (including nylon and terylene) are 'condensation polymers'.
question
Are addition polymers (e.g. polythene and PVC) biodegradable?
answer
No.
question
Are condensation polymers (e.g. nylon and terylene) biodegradable?
answer
Yes.
question
The 2 main ways of disposing of polymer waste.
answer
Landfill and incineration.
question
One benefit and one drawback of using incineration to dispose of addition polymers like polythene and polychloroethene.
answer
Benefits. The heat produced can be made use of. Less solid left to be sent to landfill. Drawbacks. Produces CO₂ that contributes to global warming. Produces toxic gases.
question
Apart from incineration and landfill, what else can be done with waste plastics?
answer
Recycling. Plastic recycling is the process of recovering scrap or waste plastic and reprocessing the material into useful products.



