A&P1 Chapter 2: Inorganic Chemistry – Flashcards
Unlock all answers in this set
Unlock answersquestion
Element
answer
Simplest form of matter to have unique chemical properties
question
Minerals
answer
Inorganic elements that are extracted from the soil by plants and passed up the food chain to humans and other organisms
question
Element Structure
answer
Atomic number: the number of protons in its nucleus Atomic weight (relative atomic mass): total of protons and neutrons in the element
question
Planetary Model
answer
A model of atomic structure similar to planets orbiting the sun
question
Atomic Structure
answer
*In the nucleus are Protons and Neutrons. -Protons have a single positive charge -Neutrons have no charge *Around the nucleus are electrons, which have a single negative charge and low mass. *Electrons swarm around the nucleus in electron shells (energy levels). The more energy an electron has, the farther away from the nucleus its orbit lies. *Electrons of the outermost shell, called valence electrons, determine the chemical bonding properties of an atom..
question
Isotopes
answer
*Varieties of an element that have a difference in neutrons/atomic mass *Many of them are unstable and decay to more stable isotopes by giving off radiation
question
Radioisotopes
answer
Unstable isotopes
question
Radioactivity
answer
Process of decay
question
Ionizing Radiation
answer
*Destroys molecules and produces dangerous free radicals and ions *Low doses= mutagenic (causing mutations in DNA) and carcinogenic (triggering cancer as a result of mutation)
question
Kinds of Radiation Produced by Nuclear Decay
answer
*Alpha Particles: Composed of 2 protons and 2 neutrons; too large to penetrate skin *Beta Particles: A free electron; can penetrate skin by a few millimeters *Gamma Rays
question
Physical Half-Life
answer
The time required for 50% of its atoms to decay to a more stable state
question
Biological Half-Life
answer
Of a radioisotope; The time required for half of it to disappear from the body
question
Ions
answer
Charged particles with unequal numbers of protons and electrons *Form because elements with one->three valence electrons tend to give them up, and those with four->seven electrons tend to gain more
question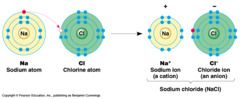
Ionization

answer
Transfer of electrons from one element to another, turning them both into ions
question
Anion
answer
Ion that gains electrons from ionization, resulting in a negative charge
question
Cation
answer
Ion that loses electrons from ionization, resulting in a positive charge
question
Octet Rule
answer
Number of electrons in outer valency shell is always 8
question
Electrolytes
answer
*Substances that ionize in water (acids, bases, or salts) and form solutions capable of conducting electricity *Important for their chemical reactivity, osmotic effects (influence on water content and distribution to the body), and electrical effects (essential to nerve and muscle function)
question
**Major Electrolytes and the Ions Released by their Dissociation**
answer
Electrolyte-> Cation and Anion ~~~ **See table 2.2 on page 46 of book, it has the chemical formulae**
question
Electrolyte Concentrations
answer
*Their electrical effects, which determine such things as nerve, hearts, and muscle actions, depend not only on their concentration but also on their electrical charge *One equivalent of an electrolyte is the amount that would electrically neutralize 1 mole of hydrogen ions or hydroxide ions *Electrolytes in our body fluids have concentrations less than 1 Eq/L, so we more often express their concentrations in milliequivalents per liter (mEq/L)
question
Free Radicals
answer
**DESTROYS MOLECULES** *Chemical particles with an odd number of electrons *Represented with a dot to symbolize the odd electron *Produced by some normal metabolic reactions of the body, by radiation, and by chemicals *Short-lived and combine quickly with molecules such as fats, proteins, and DNA, converting them into free radicals and triggering chain reactions that destroy more molecules --(EXTRA INFO)
question
Antioxidant
answer
A chemical that neutralizes free radicals
question
Molecules
answer
Chemical particles composed of two or more atoms united by a chemical bond
question
Compounds
answer
Molecules composed of two or more elements
question
Molecular Weight
answer
The sum of the atomic weights of its atoms
question
Chemical Bonds
answer
Forces that hold molecules together (attraction) *Ionic Bonds *Covalent Bonds *Hydrogen Bonds *Van Der Waals Forces
question
Ionic Bond
answer
*The attraction of a cation to an anion *Can be composed of two or more ions *Weak and easily dissociate (break up) in the presence of something more attractive
question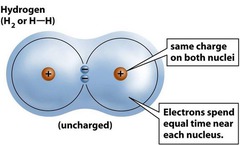
Covalent Bonds

answer
*Form by sharing electrons *Single Covalent Bond- Sharing of one pair of electrons -Symbolized by one line between atomic symbols -Bonds are strong -Formed between two non-metals *Double Covalent bond- Sharing of 2 pairs of electrons - Symbolized by double lines between atomic symbols
question
Nonpolar Covalent Bond
answer
*Shared electrons spend equal time around each nucleus *Strongest of all covalent bonds *Carbon atoms bond together using these bonds *Same # of electrons
question
Polar Covalent Bond
answer
*If shared electrons spend significantly more time orbiting one nucleus than they do the other, they lend their negative charge to the region where they spend the most time, and they form a POLAR COVALENT BOND
question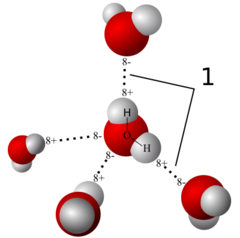
Hydrogen Bond

answer
*Weak attraction between a slightly positive hydrogen atom in one molecule and a slightly negative oxygen or nitrogen atom in another *Represented by dotted or broken lines between atoms
question
Van der Waals Forces
answer
*Weak, brief attractions between neutral atoms *Single VdWF is about 1% as strong as a covalent bond, but VdWF between large numbers of atoms can create a very strong attraction *Important in protein folding, the binding of proteins to each other and to other molecules such as hormones, and the association of lipid molecules with each other *Organic Chemistry
question
Mixture
answer
*Substances that are physically blended but not chemically combined *Each substance retains its own chemical properties
question
Water
answer
*50 to 75% of body weight *Its atoms are joined by polar covalent bonds *The molecule is V-shaped, with a 105 Degree bond angle *Molecule as a whole is polar *Properties that account for its ability to support life: Solvency, Cohesion, Adhesion, Chemical Reactivity, and Thermal Stability
question
Solvency
answer
*Ability to dissolve other chemicals *Water is the universal solvent because it dissolves a broader range of substances than any other liquid *In order to be soluble in water, a molecule must be polarized or charged so that its charges can interact with those of water
question
Solute
answer
*Can be a gas, solid, or liquid *
question
Hydrophilic
answer
Substances that dissolve in water
question
Hydrophobic
answer
Substances that do not dissolve in water
question
Cohesion
answer
Tendency of molecules of the same substance to cling to each other Ex: Water's hydrogen bonds Ex: Spilling water and having it form a puddle
question
Adhesion
answer
Tendency of one substance to cling to another Ex: Water clinging to body's tissues to form a lubricating film on membranes
question
Surface Tension
answer
*Bi-product of cohesion
question
Chemical Reactivity
answer
*Water's ability to participate in chemical reactions Ex:Water ionizes many other chemicals such as acids and salts, but water itself ionizes into H+ and OH-
question
Thermal Stability
answer
*Helps to stabilize the internal temperature of the body *Water has high heat capacity *Hydrogen bonds of water molecules inhibit their movement, so water can absorb a given amount of heat without changing temperature (molecular motion) as much *Water's high heat capacity makes it a very effective coolant
question
Solution
answer
Consists of particles of matter called the solute mixed with a more abundant substance called the solvent *Defined by following properties: -Solute particles are under 1 nanometer in size; solute and solvent cannot be visually distinguished from each other -Small particles do not scatter light noticeably, so solutions are usually transparent -Solute particles will pass through most selectively permeable membranes -Solute does not separate from the solvent when the solution is allowed to stand
question
Colloid
answer
*Most common Colloids are mixtures of protein and water, such as albumin *Can change from liquid to gel states *Defined by following properties: -Colloidal particles range from 1 to 100 nm in size -Particles this large scatter light, so colloids are usually cloudy -Particles are too large to pass through most selectively permeable membranes -Particles are still small enough to remain permanently mixed with the solvent when the mixture stands
question
Suspension
answer
*Blood cells in our blood plasma *Defined by following properties: -Suspended particles exceed 100 nm in size -Such large particles render suspensions cloudy or opaque -Particles are too large to penetrate selectively permeable membranes -Particles are too heavy to remain permanently suspended, so suspensions separate on standing
question
Emulsion
answer
A suspension of one liquid in another Ex: oil-and-vinegar salad dressing
question
Measure of Concentration
answer
A simple way to express concentration is the weight of solute in a given volume of solution
question
Molarity
answer
*High molarity if there is a lot of solute *Molarity is the number of moles of solute per liter of solution
question
Acid
answer
Any proton donor, a molecule that releases a proton (H+) in water
question
Base/Alkaline
answer
Proton acceptor
question
pH
answer
*Expresses acidity *Scale from 0-14 *A measure derived from the molarity of H+ *pH= -log[H+] *pH of 7.0 is neutral *pH below 7.0 is acidic pH above 7.0 is basic *Change of one # on pH scale represents a 10-fold change in H+ concentration; (change in 2 #s = 100)
question
Buffer
answer
A chemical solution that resists changed in pH
question
Energy
answer
Capacity to do work -Work= to move something Ex of physiological work: breaking chemical bonds, building molecules, pumping blood, contracting skeletal muscles *Potential and Kinetic Energy
question
Potential Energy
answer
Energy contained in an object because of its position or internal state but that is not doing work at the time
question
Kinetic Energy
answer
Energy of motion/energy that is doing work Ex: flow of ions into cell, vibration of eardrum, musculoskeletal movements
question
Chemical Energy
answer
Potential energy stored in the bonds of molecules *Chemical reactions release this energy to make it available for physiological work *Heat is the kinetic energy of molecular motion *Temp. of a substance is a measure of rate of this motion, and adding heat to a substance increases this rate
question
Electromagnetic Energy
answer
*The kinetic energy of moving packets of radiation called photons *Most familiar form of electromagnetic energy is light *Has both kinetic and potential forms -Potential when charged particles have accumulated at a point -Kinetic when these particles begin to move and create an electrical current
question
Free Energy
answer
Potential energy available in a system to do useful work *Most relevant free energy is the energy stored in the chemical bonds of organic molecules
question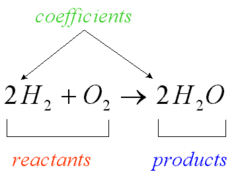
Chemical Reaction
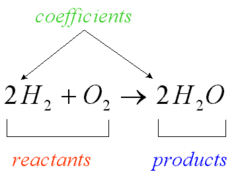
answer
Process in which a covalent or ionic bond is formed or broken *Course of a chemical reaction is symbolized by a chemical equation that typically shows reactants on the left, products on the right, and an arrow pointing from reactants to products *Classified as decomposition, synthesis, or exchange reactions
question
Decomposition Reaction
answer
A large molecule breaks down into two or more smaller ones *AB-> A + B
question
Synthesis Reaction
answer
Two or more small molecules combine to form a larger one *A + B -> AB
question
Exchange Reaction
answer
Two molecules exchange atoms or groups of atoms *AB + CD -> AC + BD
question
Reversible Reaction
answer
*Can go in either direction under different circumstances *Direction is determined by the relative abundance of substances on each side of the equation *Follow the law of mass action *Exist in a state of equilibrium; ratio of products to reactants is stable
question
Law of Mass Action
answer
Proceed from the reactants in greater quantity to the substances with the lesser quantity
question
Reaction Rates
answer
*Rate of a reaction depends on the nature of the reactants and on the frequency and force of these collisions *Factors that affection reaction rates: concentration, temperature, catalysts
question
Reaction Rate: Concentration
answer
Reaction rates increase when the reactants are more concentrated *Molecules are more crowded and collide frequently
question
Reaction Rate: Temperature
answer
*Reaction rate increases and temperature rises *Heat causes molecules to move rapidly and collide with greater force
question
Reaction Rate: Catalysts
answer
*Substances that temporarily bind to reactants, hold them in a favorable position to react with each others, and may change the shapes of reactants in ways that make them more likely to react *Releases products and is available to repeat the process with more reactants **Speeds up reaction** *Not consumed or changed by the reaction *Enzymes
question
Metabolism
answer
* *Anabolism and Catabolism
question
Catabolism
answer
Energy-releasing decomposition reactions *Reactions break covalent bonds, produce smaller molecules from larger ones, release energy *Exergonic Reactions- energy releasing reactions
question
Anabolism
answer
Consists of energy-storing synthesis reactions, such as the production of protein or fat *Endergonic Reactions- Reactions that require energy input
question
Oxidation
answer
Any chemical reaction in which a molecule gives up electrons and releases energy *Molecule becomes oxidized *Molecule that takes the electrons from it is an oxidizing agent (Electron acceptor) *Oxygen is often an electron acceptor
question
Reduction
answer
*Chemical reaction in which a molecule gains electrons and energy *Molecule is reduced when it accepts electrons *Molecule is a reducing agent (electron donor) when it donates electrons
question
Oxidation-Reduction Reactions (Redox Reactions)
answer
Electron transfers as hydrogen atoms *Oxidation of one molecule is followed by reduction of another



