Chemotherapy Drugs – Flashcards
Unlock all answers in this set
Unlock answersquestion
Chemotherapy
answer
Chemotherapy is a drug treatment that uses powerful chemicals to kill fast-growing cells in your body. Chemotherapy is most often used to treat cancer, since cancer cells grow and multiply much more quickly than most cells in the body. Chemotherapy is one of the 3 main tools we have to treat cancer (along with surgery & radiation). Often, all three established treatment modalities are used in combination to provide the patient with maximal benefit and minimal side effects. Many different chemotherapy drugs are available. Chemotherapy drugs can be used alone or in combination to treat a wide variety of cancers. Though chemotherapy is an effective way to treat many types of cancer, chemotherapy treatment also carries a risk of side effects. Some chemotherapy side effects are mild and treatable, while others can cause serious complications.
question
Radiation therapy
answer
Radiation therapy is a type of cancer treatment that uses beams of intense energy to kill cancer cells. Radiation therapy most often gets its power from X-rays, but the power can also come from protons or other types of energy. Techniques of delivering radiation include: -external beam of X-rays from a linear accelerator -implants (radioactive needles or wires implanted directly into tumor) -intracavitary technique (ex. radioactive source placed in the cervix or vagina near tumor) The term "radiation therapy" most often refers to external beam radiation therapy, where the high-energy beams come from a machine outside of your body that aims the beams at a precise point on your body. During a different type of radiation treatment called brachytherapy, radiation is placed inside your body. Radiation therapy damages cells by destroying the genetic material that controls how cells grow and divide. While both healthy and cancerous cells are damaged by radiation therapy, the goal of radiation therapy is to destroy as few normal, healthy cells as possible. note: never give radiation to kids younger than 5 years old, as their brain is still developing
question
Treatment advantages & disadvantages of surgery vs. radiation vs. chemotherapy
answer
1. Surgery Advantages: -Removes gross tumor rapidly Disadvantages: -No effect on distant metastases -Leaves microscopic cancer deposits -There are functional and cosmetic limits on the possible extent of resection 2. Radiation Advantages: -Effective against tumor masses, especially microscopic Disadvantages: -No effect on distant metastases -Large tumor masses may not be effectively treated -Damage to normal tissues within radiation treatment field 3. Chemotherapy Advantages: -Systemic distribution Disadvantages: -Often non-specific effects that damage normal cells -Large tumor masses may contain resistant tumor cells -Drug delivery limited to some tissues (ex. BBB)
question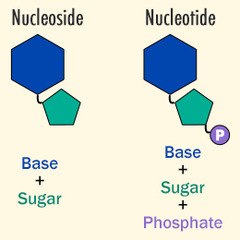
Review: Nucleotide vs. Nucleoside

answer
Nucleotides: -Adenine -Guanine -Cytosine -Thymine -Uracil Nucleosides: -Adenosine -Guanosine -Cytidine -5-Methyluridine -Uridine
question
4 Main Classes of Chemotherapy Agents
answer
1. Antimetabolites - stop tumor cells from replicating by blocking nucleotide synthesis so DNA & RNA cannot be synthesized (S-Phase) 2. Alkylating Agents - kill tumor cells by directly damaging DNA (non-phase specific) 3. Mitotic inhibitors (aka plant alkaloids) - stop tumor cells from replicating by blocking microtubule formation/disassembly so mitosis cannot occur 4. Anti-tumor Antibiotics - stop tumor cells from replicating by interfering with the DNA inside cells at multiple points in the cell cycle (some G2, some vary)
question
Overview of how chemo drugs work to stop cancer
answer
Cancerous tumors are characterized by cell division, which is no longer controlled as it is in normal tissue. "Normal" cells stop dividing when they come into contact with like cells, a mechanism known as contact inhibition. Cancerous cells lose this ability. Cancer cells no longer have the normal checks and balances in place that control and limit cell division. The process of cell division, whether normal or cancerous cells, is through the cell cycle. The cell cycle goes from the resting phase, through active growing phases, and then to mitosis (division). The ability of chemotherapy to kill cancer cells depends on its ability to halt cell division. Usually, the drugs work by damaging the RNA or DNA that tells the cell how to copy itself in division. If the cells are unable to divide, they die. The faster the cells are dividing, the more likely it is that chemotherapy will kill the cells, causing the tumor to shrink. They also induce cell suicide (self-death or apoptosis). Chemotherapy drugs that affect cells only when they are dividing are called cell-cycle specific. Chemotherapy drugs that affect cells when they are at rest are called cell-cycle non-specific. The scheduling of chemotherapy is set based on the type of cells, rate at which they divide, and the time at which a given drug is likely to be effective. This is why chemotherapy is typically given in cycles.
question
Chemo side effects
answer
Unfortunately, chemotherapy does not know the difference between the cancerous cells and the normal cells. Chemotherapy will kill all cells that are rapidly dividing. The "normal" cells will grow back and be healthy but in the meantime, side effects occur. The "normal" cells most commonly affected by chemotherapy are the blood cells, the cells in the mouth, stomach and bowel, and the hair follicles; resulting in low blood counts, mouth sores, nausea, diarrhea, and/or hair loss. Different drugs may affect different parts of the body.
question
Antimetabolites
answer
In general, these stop tumor cells from replicating by blocking nucleotide synthesis so DNA & RNA cannot be synthesized They are S-phase specific Antimetabolites are very similar to normal substances within the cell. When the cells incorporate these substances into the cellular metabolism, they are unable to divide. Antimetabolites are classified according to the substances with which they interfere. 1. Methotrexate (MTX) 2. 5-fluorouracil/capecitabine (5-FU) 3. Cytarabine (Cytosine Arabinoside) 4. Fludarabine 5. Gemcitabine Note: Myelosupression is a side effect for all of these (which makes sense because this is the point of them!) -anemia (fatigue/weakness) -thrombocytopenia (bleeding) -leukopenia (infection)
question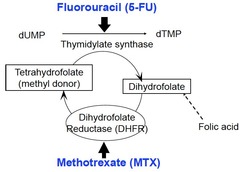
Methotrexate (MTX)

answer
Methotrexate is a Folic Acid analog Mechanism of action: blocks DNA/Protein synthesis by inhibiting dihydrofolate reductase (aka DHFR, enzyme needed to make dTMP, deoxythymidine monophosphate which is a nucleotide monomer used to make DNA) Note: Myelosuppression from Methotrexate is reversible with Leucovorin, which is chemically similar to folic acid and produces the same effect.
question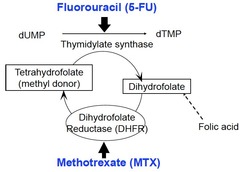
5-fluorouracil/capecitabine (5-FU)

answer
5-fluorouracil is a Pyrimidine analog (Cytosine, Thymine, Uracil) Capecitabine is a prodrug that is enzymatically converted to 5-FU) in the body Mechanism of action: blocks DNA/Protein synthesis by being activated to 5F-dUMP, inhibiting thymidylate synthase enzyme (enzyme needed to make dTMP, deoxythymidine monophosphate which is a nucleotide monomer used to make DNA).
question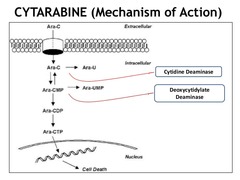
Cytarabine (aka Cytosine Arabinoside)

answer
Cytarabine is a Pyrimidine analog It is a prodrug Mechanism of action: blocks DNA/Protein synthesis by converting to its active form cytosine arabinoside triphosphate (aka ara-CTP) & getting incorporated into the DNA causing strand termination. It also inhibits DNA/RNA polymerase & Ribonucleotide Reductase enzyme (RNR), which normally catalyzes the formation of deoxyribonucleotides from ribonucleotides. Clinical use: -Acute Myeloid Leukemia (AML), standard treatment = Cytarabine + Daunorubicin (anthracycline antibiotic) Note on Pharm: short half-life, administered via IV, liver metabolism, kidney excretion
question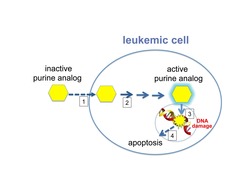
Fludarabine

answer
Fludarabine is a prodrug that gets converted to a Purine analog (Adenine, Guanine) Mechanism of action: same as Cytarabine
question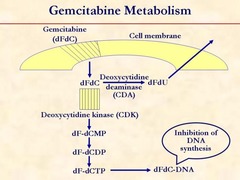
Gemcitabine

answer
Gemcitabine is a Pyrimidine analog (Cytosine, Thymine, Uracil) Mechanism of action: same as Cytarabine & Fludarabine
question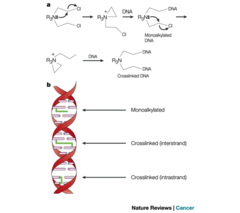
Alkylating Agents

answer
In general, these kill tumor cells by directly damaging DNA They are not phase specific Alkylating agents are a class of antineoplastic or anticancer drugs which act by inhibiting the transcription of DNA into RNA and thereby stopping the protein synthesis. Alkylating agents substitute alkyl groups for hydrogen atoms on DNA, resulting in the formation of cross links within the DNA chain and thereby resulting in cytotoxic, mutagenic, and carcinogenic effects. The end result of the alkylation process results in the misreading of the DNA code and the inhibition of DNA, RNA, and protein synthesis and the triggering of programmed cell death (apoptosis) in rapidly proliferating tumor cells. 1. Cyclophosphamide (Nitrogen Mustard) 2. Cisplatin (Platinum Complex)
question
Cyclophosphamide

answer
Cyclophosphamide is a Nitrogen Mustard trade name "Cytoxan" It is a prodrug that requires bioactivation by liver cytochrome P450 Mechanism of action: causes apoptosis by attaching an alkyl group to the guanine base of DNA, at the number 7 nitrogen atom of the imidazole ring. This interferes with DNA replication by forming intrastrand & interstrand DNA crosslinks with bulky DNA lesions. Side Effects: - Hemorrhagic cystitis: lower urinary tract symptoms that include dysuria, hematuria, and hemorrhage. (partially prevented with Mesna, where the thiol group binds toxic metabolites)
question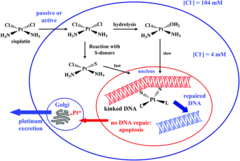
Cisplatin

answer
Cisplatin is a Platinum Complex Mechanism of action: causes apoptosis by binding to bases (preferably Guanine), which covalently cross-links DNA both interstrand & intrastrand. The damaged DNA is recognized by repair mechanisms and if the changes are beyond the scope of the cell's reparative mechanisms, then apoptosis will be triggered, leading to cell death. Clinical use: Carcinomas -Testicular -Bladder -Ovary -Lung Side Effects: - Acoustic nerve damage with bilateral hearing loss - Peripheral neuropathy
question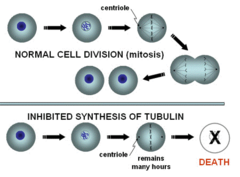
Mitotic inhibitors (aka plant alkaloids)

answer
In general, these stop tumor cells from replicating by blocking microtubule formation/disassembly so mitosis cannot occur 1. Vincristine (vinca alkaloid) 2. Paclitaxel (taxane)
question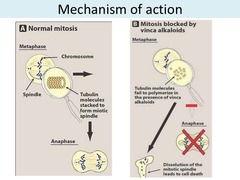
Vincristine

answer
Vincristine is a vinca alkaloid Mechanism of action: stops cell division by binding to ?- tubulin protein, which inhibits its polymerization into microtubules thereby preventing mitotic spindle formation (M-phase arrest) Side Effects: -NEUROTOXICITY due to microtubules in axons (areflexia, peripheral neuritis/neuropathy) -Paralytic ileus
question
Pacitaxel
answer
Pasitaxel is a taxane Mechanism of action: inhibits microtubule degredation preventing cell division
question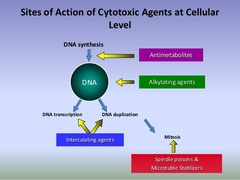
Anti-tumor Antibiotics

answer
In general, these stop tumor cells from replicating by blocking interfering with the DNA inside cells at multiple points in the cell cycle 1. Doxorubicin (Anthracycline) 2. Bleomycin 3. Irinotecan (Topoisomerase 1 inhibitor) 4. Etoposide (Topoisomerase 2 inhibitor)
question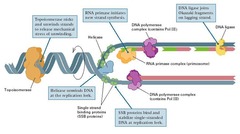
Topoisomerases

answer
enzymes that wind and unwind DNA They do this by making an incision that breaks the DNA backbone, so they can then pass the DNA strands through one another, swivelling and relaxing/coiling the DNA before resealing the breaks. DNA topoisomerases can be divided into two groups based on the number of strands that they break. Topoisomerase 1 = Break one strand of a DNA helix, ATP independent. Unwind tightly wound DNA so that it can be accessed for replication ; transcription. Topoisomerase 2 = Break two strands of a DNA helix, ATP dependent. Condense chromosomes so that DNA is not damaged when it is not being used. Since the overall chemical composition and connectivity of the DNA do not change, the tangled and untangled DNAs are chemical isomers, differing only in their global topology, thus their name ("topo" = topographical, "isomer" = same chemical structure. "ase" = enzyme) Bacterial topoisomerase and human topoisomerase proceed via the same mechanism for replication and transcription.
question
Doxorubicin

answer
Doxorubicin is an Anthracycline non-cell cycle specific Trade name = "Adriamycin" Mechanism of action: 1. Inhibits DNA transcription by intercalating (aka inserting a molecule) into the DNA 2. Prevents DNA replication by blocking Topoisomerase II after it has broken the DNA chain for replication, so the DNA stays unwound 3. Damages DNA by generating free ROS Side Effects: -Cardiotoxicity (dose related), specifically dilated cardiomyopathy (prevented with iron chelating agent Dexrazoxane) Note on Pharm: liver metabolism (must be adjusted in patients with hepatic dysfunction)
question
Bleomycin

answer
G2 phase specific Mechanism of action: Damages DNA by creating ROS that break strands of DNA Side Effects: -Pulmonary fibrosis (contraindicated with pre-existing lung disease)
question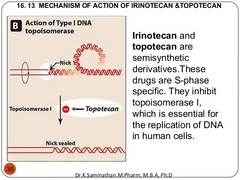
Irinotecan

answer
act in G2 ; S-phase prodrug that is metabolized to it's active metabolite Mechanism of action: prevents prevents DNA unwinding and replication by blocking Topoisomerase 1 from religating the DNA strand breaks. Thus, DNA strand breaks accumulate and will ultimately trigger apoptosis. Clinical use: -Colon cancer (metastatic)
question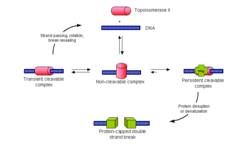
Etoposide

answer
act in G2 & S-phase Mechanism of action: Causes DNA damage & apoptosis by blocking Topoisomerase 2 religation of the breaks in DNA which occur when the DNA coils unwind. This DNA damage will result in activation of apoptotic pathways causing cell death.
question
Other Chemotherapy Agents
answer
1. Proteasome inhibitor - kills tumor cells by inhibiting the proteasome, which leads to apoptosis (ex. Bortezomib) 2. Thalidomide derivative - stops tumors from growing by blocking immune cytokines TNF-? & IL-6 so there is no stimulation for angiogenesis or for WBC to replicate in the bone marrow (ex. Lenalidomide) 3. Small molecule inhibitors - small molecules that can enter tumor cells and stop them from replicating by blocking tyrosine kinases so growth factor receptors cannot respond to stimulation (ex. Imatinib, Sorafenib, Erlotinib) 4. Monoclonal antibodies - kills tumor cells by targeting specific proteins that are expressed on different types of tumors (Ex. Rituximab, Trastuzumab, Bevacizumab, Cetuximab) 5. Immune checkpoint inhibitors - kills tumor cells by activating the immune system (by blocking specific proteins that usually block the immune response) so it can attack tumor cells (ex. Ipilimumab, Nivolumab)
question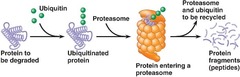
Review: Proteasome

answer
The function of the proteasome is to degrade ubiquitylated proteins This produces peptides which can be combined with MHC molecules as part of the immune response
question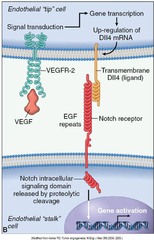
Review: VEGF

answer
VEGF = Vascular Endothelial Growth Factor (VEGF) It is a signal protein produced by cells that stimulates vasculogenesis (the de novo formation of the embryonic circulatory system) and angiogenesis (the growth of blood vessels from pre-existing vasculature). It is part of the system that restores the oxygen supply to tissues when blood circulation is inadequate. All members of the VEGF family stimulate cellular responses by binding to tyrosine kinase receptors (the VEGFRs) on the cell surface of vascular endothelium of blood vessels, causing them to dimerize and become activated through transphosphorylation. VEGF's normal function is to create new blood vessels during embryonic development, new blood vessels after injury, muscle following exercise, and new vessels (collateral circulation) to bypass blocked vessels. When VEGF is overexpressed, it can contribute to disease. Solid cancers cannot grow beyond a limited size without an adequate blood supply; cancers that can express VEGF are able to grow and metastasize. Overexpression of VEGF can cause vascular disease in the retina of the eye and other parts of the body. Serum concentration of VEGF is high in bronchial asthma and diabetes mellitus. Drugs such as bevacizumab and ranibizumab can inhibit VEGF and control or slow those diseases.
question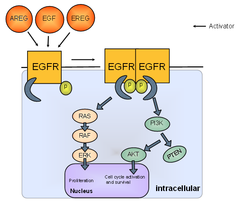
Review: EGF

answer
EGF = Epidermal Growth Factor It is a protein hormone that stimulates cell growth, proliferation, and differentiation EGF acts by binding with high affinity to epidermal growth factor receptor (EGFR) on the cell surface. This stimulates ligand-induced dimerization, activating the intrinsic protein-tyrosine kinase activity of the receptor (see the second diagram). The tyrosine kinase activity, in turn, initiates a signal transduction cascade that results in a variety of biochemical changes within the cell that ultimately lead to DNA synthesis and cell proliferation These include: -a rise in intracellular calcium levels -increased glycolysis and protein synthesis -increases in the expression of certain genes including the gene for EGFR HER2/neu is a member of the epidermal growth factor receptor family and is overexpressed in about 15-20% of breast cancers.
question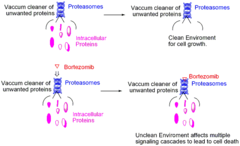
Bortezomib

answer
Mechanism: Proteasome inhibitor that kills tumor cells by inhibiting the proteasome, which leads to apoptosis. The boron atom in bortezomib binds tightly to the catalytic site of the 26S proteasome and inhibits its function. There are a number of postulated mechanisms by which this results in the relative specificity for killing tumor cells but are beyond the scope of this course. Clinical use: -Multiple Myeloma (; Mantle Cell Lymphoma, a BCNHL)
question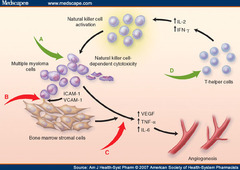
Lenalidomide

answer
Mechanism: Thalidomide derivative that stops tumors from growing by blocking immune cytokines TNF-? ; IL-6 so there is no stimulation for angiogenesis or for WBC to replicate in the bone marrow (while also activating some other immune cells to kill the cancer) Clinical use: -Multiple Myeloma (; Myelodysplastic Syndrome)
question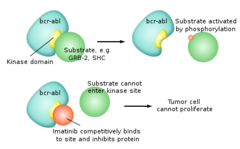
Imatinib

answer
Mechanism: Blocks cell proliferation by inhibiting a few different tyrosine kinases (bcr-abl from the Philadelphia chromosome fusion gene in CML) ; also c-Kit common in GI solid tumors). Imatinib sits in the pocket of the tyrosine kinase enzyme at the site where ATP binds. This prevents the transfer of a phosphate group to other proteins and hence blocks downstream signaling pathways. Clinical use: -CML ("Philadelphia chromosome") -GI stromal tumors
question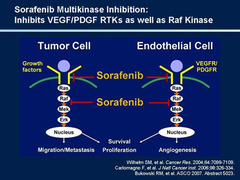
Sorafenib

answer
Mechanism: blocks angiogenesis and tumor growth by inhibiting tyrosine kinases (including VEGFR ; Raf kinase)
question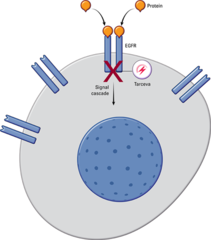
Erlotinib

answer
Mechanism: blocks cell proliferation and protein synthesis by inhibiting EGFR tyrosine kinase. It is a reversible inhibitor which attaches to the ATP binding site. The drug has a higher affinity for the mutated form of EGFR which has rendered it constitutively activated and is thus more active in patients whose malignancy has this genetic abnormality. Clinical use: (EGFR+ non-small cell lung cancer)
question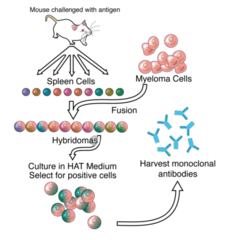
Monoclonal Antibodies production and complications

answer
These medications are generally well tolerated with few/rare side effect (but are expensive) Injection of a non-human protein into a human will result in an immune response which can include production of a human antibody against the murine antibody which would neutralize its action. More significant reactions include serum sickness and anaphylaxis. For all these reasons, investigators developed methods to greatly reduce the proportion of mouse antigen (chimerization) or eliminate it (humanization). Different monoclonal antibodies have an anti-tumor effect by different mechanisms as is described for each specific medication.
question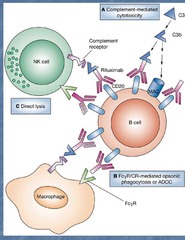
Rituximab

answer
Mechanism: antibody that kills mature B-cells by binding to CD20, causing direct lysis, induced apoptosis, and complement binding-induced death Clinical use: -B-cell cancers: CLL, HL, NCBCL -Rheumatoid arthritis (with Methotrexate) -Idiopathic thrombocytopenic purpura (ITP) Side effects: -Allergic reaction due to chimeric mouse/human antibody -Tumor lysis syndrome (when malignant lymphocytes are ; 25k) -Reactivation of Hepatitis (HBV)
question
Tumor lysis syndrome

answer
a group of metabolic disturbances that may follow the initiation of cancer treatment. It usually occurs in patients with bulky, rapidly proliferating, treatment-responsive tumors. Although tumor lysis syndrome has been reported with virtually every type of tumor, it is typically associated with acute leukemias and high-grade non-Hodgkin lymphomas, such as Burkitt lymphoma. A potentially lethal complication of anticancer treatment, tumor lysis syndrome occurs when large numbers of neoplastic cells are killed rapidly, leading to the release of intracellular ions and metabolic byproducts into the systemic circulation. Clinically, the syndrome is characterized by rapid development of hyperuricemia, hyperkalemia, hyperphosphatemia, hypocalcemia, and acute renal failure. The main principles of tumor lysis syndrome management are: (1) identification of high-risk patients with initiation of preventive therapy (2) early recognition of metabolic and renal complications and the prompt administration of supportive care, including hemodialysis.
question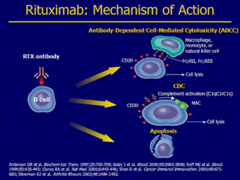
Rituximab Diagram

answer
Note: Ab are phagocytosed and catabolized in the reticuloendothelial system.
question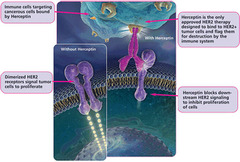
Trastuzumab

answer
Mechanism: antibody that blocks proliferation of breast cancer cells by 3 mechanisms: (1) binding to EGF-R encoded by HER-2 and blocking dimerization/cellular signaling (2) down regulation of gene expression of EGFR (3) Ab-dependent cell death Clinical use: -HER-2+ breast cancer Side effects: -Cardiotoxicity "HER damages the HEART"
question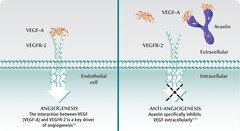
Bevacizumab

answer
Mechanism: antibody that stops progression of cancer cells by binding VEGFA (protein hormone) and blocking angiogenesis Clinical use: -k-ras+ colon, pancreatic, ; RCC
question
Cetuximab

answer
Mechanism: antibody that stops proliferation of cancer cells by binding EGF-R preventing dimerization Clinical use: -k-ras- colon cancer
question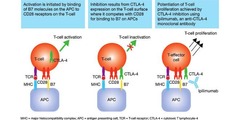
Ipilimumab

answer
Mechanism: antibody that activates killer T-cells to kill cancer cells (via binding/inhibition of CTLA-4 receptor which normally inhibits them) Clinical use: -Melanoma
question
Specific Cancer Treatments
answer
1. Acute promyelocytic leukemia (ex. All Trans Retinoic Acid ; Arsenic trioxide) 2. Prostate cancer (ex. Leuprolide , Bicalutamide, Abiraterone) 3. Breast cancer (ex. Tamoxifen, Anastrozole)
question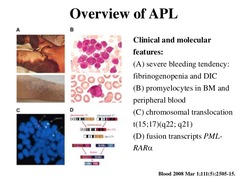
Acute Promyelocytic Leukemia drugs (AML subtype 3)

answer
Tretinoin (aka "all trans retinoic acid") - blocks the action of PML-RAR gene product so that promyelocytes can mature properly Arsenic trioxide - toxic old chinese remedy that induces cancer cells to undergo apoptosis
question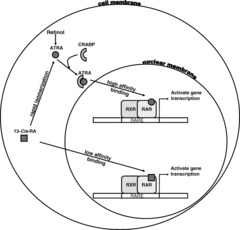
All Trans Retinoic Acid

answer
This works in APL because the majority of cases involve a chromosomal translocation of chromosomes 15 and 17, which causes genetic fusion of the retinoic acid receptor (RAR) gene to the promyelocytic leukemia (PML) gene. This fusion PML-RAR protein is responsible for preventing immature myeloid cells from differentiating into more mature cells. This block in differentiation is thought to cause leukemia. All Trans Retinoic Acid acts on PML-RAR to lift this block, causing the immature promyelocytes to differentiate to normal mature blood cells thus decreasing promyelocytes.
question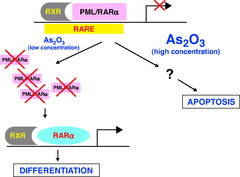
Arsenic trioxide

answer
old chinese medicinal remedy that induces cancer cells to undergo apoptosis. It is a second line medication after All Trans Retinoic Acid. Due to the toxic nature of arsenic, this drug carries significant risks.
question
Prostate cancer drugs

answer
1. Leuprolide 2. Bicalutamide 3. Abiraterone
question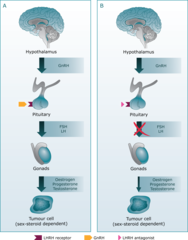
Leuprolide

answer
Mechanism: irreversible GnRH agonist which blocks proliferation of cancer cells by initially stimulating then paradoxically blocking release of LH/FSH (thus Testosterone) due to receptor down regulation of GnRH-R. Due to the constant as opposed to normal pulsatile stimulation, the receptors become downregulated and secretion of luteinizing hormone (LH) and follicle stimulating hormone (FSH) are decreased. Over two to four weeks the levels of testosterone drop to below castrate levels. Uses: -Prostate cancer -It is also used in treating women with estrogen dependent breast cancer
question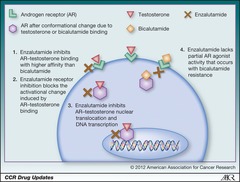
Bicalutamide

answer
Mechanism: blocks proliferation of cancer cells by competitively binding/inhibiting androgen-R, preventing testosterone binding and stimulation of cell growth Uses: -Prostate cancer (used in combination with Leuprolide to prevent initial LH ; FSH surge)
question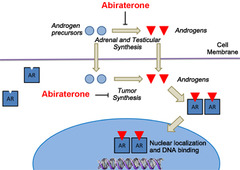
Abiraterone

answer
Mechanism: blocks proliferation of cancer cells by inhibiting enzyme CYPc17 (17 ?-hydroxylase/C17,20-lyase) which is necessary to produce testosterone precursor DHEA. This enzyme is produced in testicular, adrenal and prostatic tissue and is involved in Castration-Resistant Prostate Cancer cell self-synthesis of androgens. Uses: -Metastatic Castration-Resistant Prostate Cancer
question
Breast cancer drugs
answer
1. Tamoxifen 2. Anastrozole
question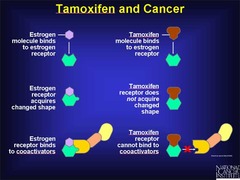
Tamoxifen

answer
Mechanism: Selective Estrogen-R modulator (SERM) that blocks proliferation of cancer cells by binding Estrogen-R in the breast (while stimulating Estrogen-R in the uterus ; bone, causing proliferation of cells in those tissues) Uses: -Estrogen/Progesterone-R+ breast cancer (ER+/PR+ ; ER-/PR+ ; ER+/PR-) -5 years of treatment Side Effects: -increased risk of endometrial cancer (1/1000)
question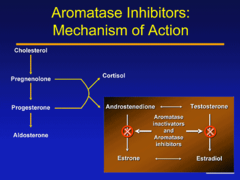
Anastrozole

answer
Mechanism: blocks proliferation of cancer cells by decreasing serum [estrogen] by inhibiting aromatase enzyme that converts testosterone to estradiol ; androstenedione to estrone Uses: -POSTMENOPAUSAL women with hormone-R+ breast cancer
question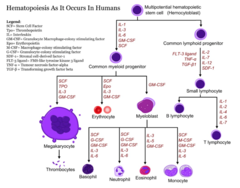
Hematopoietic growth factors

answer
1. Erythropoietin: stimulatory hormone for growth of RBC (erythrocytes) 2. Filgrastim: G-CSF (Granulocyte-Colony Stimulating Factor) analogue produced by recombinant DNA technology in e. Coli
question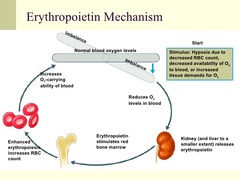
Erythropoietin

answer
Mechanism: a hormone normally secreted by the kidneys that binds to erythropoietin receptor (EpoR) on the bone marrow erythroid progenitor cells and activates a JAK2 signaling cascade. This normally works to increase the rate of production of red blood cells in response to falling levels of oxygen in the tissues. Uses: -Anemia resulting from various diseases, including chemotherapy and radiation Side Effects: -thromboembolism -PE -Stroke -MI
question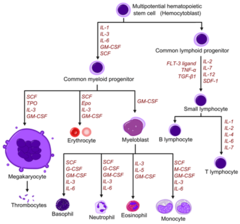
Filgrastim

answer
Mechanism: a hematopoietic growth factor which is an analogue of G-CSF (Granulocyte-Colony Stimulating Factor), which stimulates the production ; maturation of PMNs Uses: 1. to decrease the time of leukopenia (low WBC count) after chemotherapy 2. Mobilization of Hematopoietic Stem Cells to the peripheral blood for harvest prior to Stem Cell transplant Side Effects: **Severe dyspnea



