wk12-Genetics of colorectal cancer – Flashcards
Unlock all answers in this set
Unlock answersquestion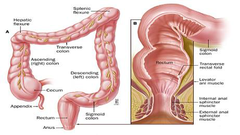
What is colorectal cancer(CRC)?

answer
-colorectal cancer is cancer in the colon and the rectum
question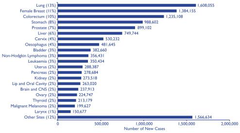
Cancer prevalence

answer
-1/3 ppl develop cancer during their lives •1/250 men •~1/300 women diagnosed each year •slightly more men than women -CRC=3rd most prevalent cancer -1/40 ppl in the developed countries of Western Europe and North America will develop the bowel or colon
question
Why is colorectal cancer the easiest to prevent?
answer
•heavily influenced by diet and a lot of environmental factors •colonoscopy to detect it early→easiest way to detect it
question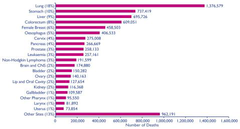
Cancer mortality

answer
->1/4 die from cancer -cancer accounts for : •30% of all deaths in males •25% of all deaths in females -CRC is the 4th most common cause of cancer death -when detected early we can take it out surgically and then its a cure -if it has metastasised then there is a problem and most likely leads to death
question
Epidemiology
answer
-Peak incidence at 60-70 yrs -< 20% cases before 50 yrs -Males affected~20% more often than males -Adenomas(polyps)-presumed benign precursor lesions for most cancers -good images of polyps can be obtained from colonoscopy
question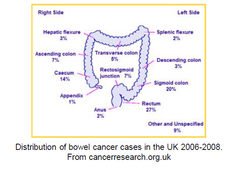
Distribution of bowel cancer cases in the UK 2006-2008

answer
-most found in rectum and lower part -the rectum is relatively accessible to tumour cells -other parts have smaller regions that need a colonoscopy
question
Risk factors
answer
-Genetic: •inflammatory bowel disease(ulcerative colitis) or polyposis syndrome •hereditary nonpolyposis colorectal cancer syndrome (HNPCC, lynch syndrome) >can get polyps but not as numerous as FAP -Environmental influences: •Dietary: >low content of unabsorbable vegetable fibre >high content of refined carbs and high fat content >low intake of protective micronutrients(vitamins A, C, and E) >red meat and processed bacon •Obesity and lack of exercise •Chemical carcinogens: components of tobacco smoke and alcohol •Use of aspirin and other NSAIDs: >protective effect against colon cancer >cyclooxygenase-2 & prosaglandin E
question
Symptoms of colorectal cancer-Red flag symptoms
answer
-bleeding from the rectum or blood in the stools >not all bleed -change in normal bowel habits to diarrhoea/looser stools lasting longer than 4-6 wks >constipation→diarrhoea -a lump in the rectum/abdomen(more commonly on the right side) -a feeling of need to strain -loss of weight without trying -pain the abdomen/rectum -anaemia→tiredness and sometimes breathlessness -cancer can cause bowel obstruction leading to: •griping pains in the abdomen •feeling bloated •constipation •vomiting
question
Benign vs malignant
answer
-Benign tumour: •slow growing •well differentiated cells •confined to normal location •good prognosis -Malignant tumour: •fast growing •poorly differentiated cells •invaded surrounding tissue •distant metastasis •poor prognosis
question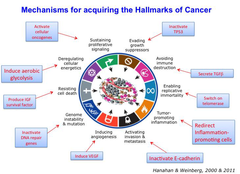
Mechanisms for acquiring the Hallmarks of Cancer

answer
-Hanahan and Weinberg 2011: Hallmarks of Cancer •Angiogenesis: development of new blood vessels by tumour to prevent hypoxia 1•Sustaining proliferative signalling→Activate cellular oncogenes 2•Evading growth suppressors→Inactivate TP53 3•Avoiding immune destruction→Secrete TGFβ 4•Enabling replicative immortality→Switch on telomerase 5•Tumor- promoting inflammation→ Redirect Inflammation- promoting cells 6•Activating invasion & metastasis→Inactivate E-cadherin 7•Inducing angiogenesis→Induce VEGF 8•Genome instability & mutation→Inactivate DNA repair genes 9•Resisting cell death→Produce IGF survival factor 10•Deregulating cellular energetics→ Induce aerobic glycolysis
question
Survival and incidence of CRC stage
answer
-if detected early its a cure -Duke's D-work in 30s-poor survival-never described by Duke as it's a new stage that has been implemented→he didn't known about later distant metastasis -want to identify early to cure
question
Screening for sporadic CRC
answer
-design to identify CRC early→improving survival rates as surgery usually curative for Stage 1 •Fecal Occult Blood Test(FOBT): test for blood in the stool→patient has to take own stool •Flexible sigmoidoscopy: rectum and sigmoid colon are visually inspected→detects about 65%-75% of polyps and 40%-65% of CRCs •if something is found a full colonoscopy is done
question
Staging of colorectal cancer
answer
-staging= the stage of cancer means how big it is and whether it has spread •tests and scans done to the patient when diagnosing the cancer give some information about the stage •impt. as treatment is often based on the stage of a cancer 2 staging systems: 1-TNM: commonly used in the UK→gives a detailed description of the size of the tumour and whether it has spread to nearby lymph nodes or another part of the body 2-Duke's system: divided into 4 grps-A,B,C and D→A is early and D is advanced
question
Duke's system

answer
-divided into 4 grps: A-D •A is early and D is advanced -Duke's A: •cancer is only in the innnermost lining of the bowel or slightly growing into the muscle layer -Duke's B: •cancer has grown through the muscle layer of the bowel -Duke's C: •cancer has spread to at least one lymph node in the area close to the bowel -Duke's D: •cancer has spread to somewhere else in the body e.g. liver or lungs -at first only included 3 stages but was later changed to include Duke's D→some prefer to call it spread stage 4 or advanced bowel cancer
question
TNM stage system
answer
-TNM=Tumour Node Metastases -doctor puts the T,N,M results together to give u your stage -T(tumour): describes the size of the primary tumour •4 stages of tumour size in bowel cancer: >T1→tumour only in inner layer of the bowel >T2→tumour grown into muscle layer of the bowel wall >T3→tumour has grown into the outer lining of the bowel wall >T4→tumour has grown through the outer lining of the bowel wall→grown into another part of the bowel or to nearby organs -N(node): describes whether any lymph nodes contain cancer cells •3 stages of whether cancer cells are in the lymph nodes: >N0→no lymph nodes with cancer cells >N1→1-3 lymph nodes close to bowel contain cancer cells >N2→cancer cells in 4 or more nearby lymph nodes -M(metastases): whether the cancer has spread to another part of the body •2 stages: >M0→cancer has not spread to other organs >M1→the cancer has spread to other parts of the body
question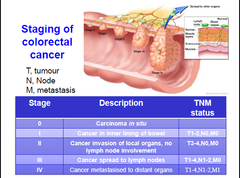
Number system

answer
-number system uses the TNM stages to group bowel cancers -5 main stages in the system: 1-Stage 0-Carcinoma in situ(CIS): •cancer cells present in the inner bowel lining but all are contained within this lining→little risk of any cancer cells having spread 2-Stage 1-Bowel cancer •cancer has grown through inner lining of the bowel or into the muscle wall but no further •no cancer in lymph nodes •TNM=T1,N0,M0 or T2,N0,M0 3-Stage 2-bowel cancer-divided into 2a and 2b 2a=cancer has grown into outer covering of bowel wall but no cancer cells in lymph nodes (T3,N0,M0) 2b=cancer has grown through outer covering of the bowel wall and into tissues or organs next to the bowel but no cancer cells in the lymph node, and it has not spread into another area of the body (T4,N0,M0) 4-Stage 3- bowel cancer-divided into 3 stages 3a: cancer still in inner layer of bowel wall or grown into muscle layer but 1-3 lymph nodes have cancer cells(T1,N1,M0 or T2,N1,M0) 3b:cancer has grown into the outer lining of the bowel wall or surrounding body tissues/organs and 1-3 lymph nodes contain cancer cells(T3,N1,M0 or T4, N1,M0) 3c: cancer can be any size and has spread to 4 lymph nodes but not to other parts of the body(any T, N2,M0) 5-Stage 4-bowel cancer •cancer has spread to other parts of the body through the lymphatic/blood system(any T, any N, M1)
question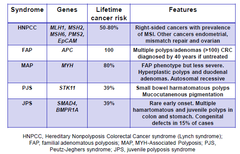
Features of inherited CRC syndromes

answer
-make up a small # of patients •FAP •MAP=less severe FAP phenotype >hyperplastic >duodenal adenomas >autosomal recessive •PJS =exon deletion→hermatomatous polyps→muscles •JPS- occurs in both colon and stomach→congenital defects in 15% of cases -APC and MYH are classic TSG genes
question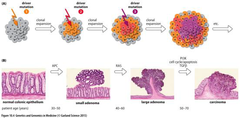
Driver DNA Mutations in the multistage evolution of colorectal cancer

answer
-mutations in germline and tumour DNA identified by sequencing DNA -epigenetic mutation e.g. methylation are also important -multistage evolutionary process -end up with genetically heterogenous tumour in terms of mutations -key mutations are the APC→RAS mutation→another adenoma -cells have a selective advantage
question
2 main types of cancer genes
answer
1-Loss of function→TSGs 2-gain of function→proto-oncogenes/oncogenes -both mutated during CRC development
question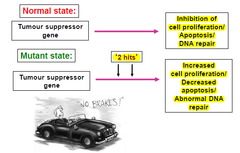
Tumour suppressor genes

answer
-discovered by the studies of hereditary cancer in humans e.g. retinoblastoma → Harris and co in late 1960s -initially referred to as anti-oncogenes -constitute the largest group of cloned hereditary cancer genes -a germline mutation in a TSG does not by itself provoke carcinogenesis→more somatic mutation at one or more loci is necessary and environmental factors e.g ionizing radiation may be significant -20 TSGs have been identified -need two alleles to be mutated →absence of gene product in the homozygous state leads to development of the particular tumour -function as a class of cellular genes that suppress inappropriate cell proliferation(development of malignancy due to LOF mutation)
question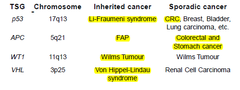
Deactivation of TSGs

answer
-LOF of one allele of a gene in which the other allele was already inactivated by: •mutation leading to loss of protein function •gene deletion •loss of heterozygosity
question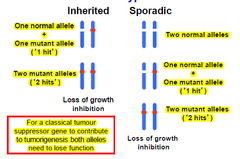
The two-hit hypothesis

answer
-1971-Knudson carried out an epidemiological study of a lrg # of cases of both types of Rb → proposed the "two-hit" hypothesis to explain the occurrence of this rare tumour in patients with and w/o a +ve family history •affected individuals with a +ve family history(hereditary form): inherited one non-functional gene present in all cells (germline mutation)→second gene at the same locus becomes inactivated somatically in a developing retinal cell >occurrence of second mutation is likely given the lrg # of retinal cells→explains AD inheritance >hereditary Rb tumours are often bilateral and multifocal >although Hereditary Rb follows an AD pattern of inheritance at the molecular level it is recessive→tumour only occurs after the loss of both alleles •non-heritable/sporadic form: 2 inactivating somatic mutations need to occur independently in the same retinoblast cell→less likely to occur >tumours in these patients are unilateral and unifocal >tumours usually occur at a later age than in the hereditary form >genes usually found in inherited and then tested in sporadic
question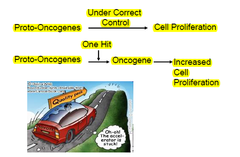
Proto-Oncogenes/Oncogenes

answer
-oncogenes: altered forms of normal proto-oncogenes -proto-oncogenes: normal genes that have key roles in cell growth and differentiation pathways •normal mammalian cells=sequences of DNA that are homologous to viral oncogenes=proto-oncogenes/cellular oncogenes •proto-oncogene=normal gene •cellular oncogene(c-onc)= mutated proto-oncogene that has oncogneic properties e.g. viral oncogenes(v-onc) -50 oncogenes have been identified
question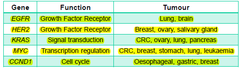
Activation of proto-oncogenes/oncogenes

answer
-mutation→change in protein function/regulation -gene duplication -chromosomal translocation -abnormal expression→e.g. increased expression of eostrogen -effect is usually dominant at the cellular level
question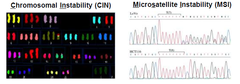
Genotyping sporadic colorectal cancers

answer
-CRCs=heterogeneous grp of diseases -no two cancers are exactly the identical -Molecular genotypes have been delineated based on distinctive patterns of genetic instabilities including: 1-Chromosomal instability (CIN)= imbalance in chromosome #s/ cell→rearrangements occur resulting in gene deletions + amplification -a type of genomic instability in which chromosomes are unstable→either whole chromosomes or parts of chromosomes are duplicated or deleted →unequal distribution of DNA to daughter cells in mitosis →failure to maintain euploidy→aneuploidy -seen mostly in APC mutations -common occurrence in solid and haematological cancers, especially colorectal cancer -seen in both inherited and sporadic 2-Microsatellite instability (MSI)=changes in #s of repeats caused by loss of mismatch repair proteins→can cause mutations in gene coding sequences e.g. TGFβRII, IGFIIR, MSH3,MSH6,BAX, MBD4 -caused by changes in mismatch repair proteins →changes in number of repeats -mutually exclusive -in HMPCC -19-20% of CRC will be based on microsatellite instability
question
How do we genotype CRCs?
answer
-Microsatellite analysis: •markers=BAT25, BAT26 •18% of bowel cancers show MI •stable-tumour has extra peak in comparison with the normal DNA control •instability- seen as an unequivocal extra peak shift compared with normal -CIN Ploidy analysis: •Ploidy and LOH analysis using 8 markers •diagnosed using analytical techniques at the cellular level: >cytogenetics >flow cytometry >Comparative genomic hybridization >Polymerase Chain Reaction >Karyotyping >fluorescence in situ hybridization (FISH) •stable-only one peak •aneuploid-has abnormal number of chormosomes=new peak
question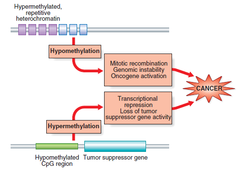
Somatic changes to Methylation status of tumour

answer
-red=methylated regions -green=unmethylated regions -methylation causes suppression of gene expression (silenced gene expression) and maintains stability of the genome→especially in areas where there are is a lot of repetitive DNA(heterochromatin)→might otherwise become erroneously involved in recombination events→altered regulation of adjacent genes Relevance to cancer: -can see hypo/hypermethylation in a cancer gene Hypomethylation: -emerged in 1983: studies showed that the genomes of cancer cells where hypomethylated compared with normal cells especially in repetitive DNA •LOI(loss of imprinting) may lead to activation of an allele that is normally silent→high expression of a product that confers advantageous cellular growth •appears to be an early event in many cancers→may correlate with disease severity Hypermethylation: -hypomethylated stretches of CpG sequence becomes methylated→ transcriptional suppression of TSG and cell regulatory genes
question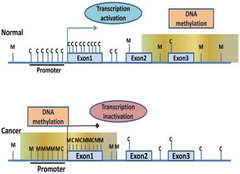
The CpG island methylator phenotype (CIMP)

answer
-CIMP CRCs have specific features: •proximal tumour location - at top of the bowel •higher BRAF and lower TP53 mutation rates -studies on going to identify specific CIMP associated methylation profile •diff. methylation phenotype in Bangladeshi community -unclear how to define and further sub-characteristic CIMP CRCs cases→unclear how to use markers
question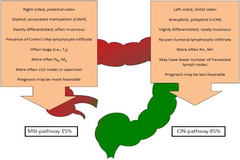
MSI and CIN-pathway CRC have different features

answer
-often miscegenous -prognosis more favourable -MSI pathway(15%)-Right sided proximal CRC: •more common in women •derived from midgut •CIMP+,BRAF mutation •MAPK signalling, serrated pathway •Mutagenic CYP450 metabolites •HPNCC •caused by increased fat and carb levels -CIN pathway(85%)-Left sided(distal) CRC: •more common in men •derived from hindgut •APC, K-ras, DCC, p53 mutations •EGFR signalling, Wnt signalling •HER1,HER2 amplification •FAP •caused by increased meat and protein and decreased calcium diets
question
Frequency of Microsatelite and Chromosome Stable cancers

answer
-not all cancers show clear MSI-CIN phenotype -MSI-CIN→microsatellite and chromosomal stable(MACS) •a percentage (17-50%) of CRCs were found to be neither CIN nor MSI→were called as microsatellite- and chromosomal-stable (MACS) CRCs •show some features of CIN phenotype •show less chromosomal gross rearrangements -Samples: •N=170 matched normal and tumour tissue •No FH of disease •DNA extracted following H&E directed dissection -MACS=1/3 of colorectal cancer
question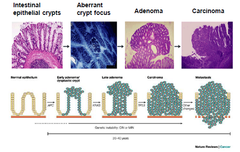
Progression of Sporadic CRC

answer
-APC→KRAS→metastasis -CIN→chromosomal instability 1-colorectal cancer is thought to be initiated by inactivation of the adenomatous polyposis coli (APC) TSG pathway in a cell somewhere within the colon The significance of unstable chromosomes in colorectal cancer •85% of cases APC gene mutated and rest β-catenin protein mutated→both have same physiological effects 2-crypt in which APC mutant cell resides becomes dysplastic as abnormal cells accumulate slowly to produce a polyp 3-development of large polyp requires acquisition of further mutations→KRAS or BRAF oncogenes 4-10-20% of large polyps will progress to cancer by acquiring additional mutations in genes of the TGF-β pathway, the p53 pathway and other pathways 5-individual cells within the bulk population that acquire such mutations are clonally selected on basis of improved fitness→bottleneck in development of cancer→advantageous mutations within this cell become fixed in future generations •tumours are heterogeneous because of continuing increase of genetic changes -Whole process from occurence of first APC mutation→development of metastatic cancer generally takes 20-40yrs→GI develops at some point during this time
question
Step-wise model of colorectal cancer tumorigenesis
answer
-develop over course of 20-40yrs -due to episodic increase of specific mutations in oncogenes such as KRAS and TSGs such as APC and TP53 -mutations arise within the tumour in a characteristic sequence -single cell within heterogeneous pop. acquires a mutation in one gene→mutation gets fixed because of growth advantage provided to cell by mutation -GI is thought to occur somewhere during the process of colorectal tumorigenesis to accelerate the rate of mutation in dividing cancer cells -evidence 4 CIN presence is limited due to small ss -strong evidence that CIN occurs early on in tumorigenic process→don't know if first event/b4 APC yet
question
2 of major pathways from adenoma to carcinoma in CRC?
answer
1-CIN 2-MSI
question
Inherited or sporadic colorectal cancer?:Clues to inherited cancer
answer
-disease appears to cluster within the family -age of onset is lower in the family than in general population -disease is inherited in a Mendelian manner -Penetrance-frequency of a phenotype associated with a mutation
question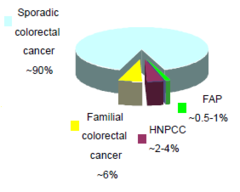
Inherited CRC

answer
-~5-10% of cancer due to an inherited germline mutation -identification of at-risk individuals is impt. due to high lifetime risk of developing cancer in gene carriers -2 well-characterised, rare Mendelian predispositions to CRC: •Familial anemoatous polyposis(FAP) •Hereditary nonpolyposis colorectal cancer (HPNCC)
question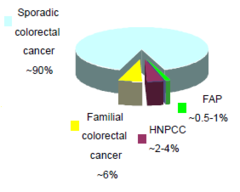
Inherited and Sporadic CRC: Sporadic CRC

answer
-mutations are somatic and acquired -studying rare CRC syndromes→understanding the genetic basis of sporadic CRC
question
Adenomatous polyposis coli (APC) as a TSG
answer
->80% tumours show inactivation of APC -APC mutation are early and very frequent event in CRC -majority of mutations inactive the gene -component of Wnt/b-catenin signalling pathway
question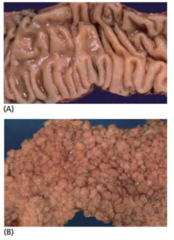
Familial adenomatous polyposis (FAP)

answer
-1% of ppl who develop colorectal cancer develop it through inheritance of an AD disorder known as FAP -affected ppl develop numerous polyps of the large bowel→can involve its entirety •carpet of polyps not all but some develop cancer •remove bowel of patients in late 20 -high risk of carcinomatous change taking place in these polyps→>90% of ppl with FAP develop bowel cancer -interstitial deletion of a particular region of the long arm of chromosome 5(5q21)→linkage of FAP to DNA markers→APC gene -inherit a single mutant APC alelle -acquire a 2nd hit over time -100% penetrant -100% CRC risk
question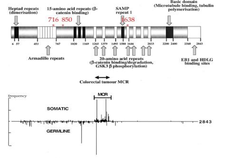
APC mutations

answer
-~300 diff. genetic mutations •point mutations •small insertions and deletions -large deletions=~15% -promoter hypermethylation -very common in sporadic CRC -most lead to truncated protein: •most impt. feature=B-catenin binding site •truncated mutations stops rest of protein being produced
question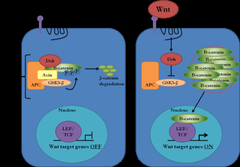
APC and Wnt pathway

answer
Wnt off: •β-catenin complexes to APC, axin and GSK-β •β-catenin is phosphorylated→targeted for degradation •No Wnt target genes are transcribed Wnt on: •β-catenin uncouples from degradation complex and accumulates in the cytoplasm •β-catenin translocates to nucleus→complexes with Lef/Tcf TFs→activate target oncogenes
question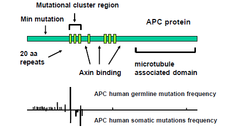
APC functional domains and mutations

answer
-all mutations in 20aa repeat region -slightly higher rate of APC human germline mutations compared to APC human somatic mutations
question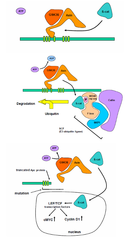
Truncated APC protein

answer
-if the APC protein is truncated -lock and key structure doesn't form -β-catenin cannot enter and bind with axin -β-catenin cannot be degraded and so it goes into the nucleus to activate TFs→overexpression and tumours
question
Hereditary Non-polyposis colorectal cancer (HNPCC)
answer
-also called Lynch Syndrome -polyps may be present but in smaller #s -average age of onset=mid forties -inherited as an AD disorder -site-specific colonic cancer: occur ore frequently in the proximal, or right side, of the colon -risk of small intestinal cancers including stomach, endometrial cancer(60%) and a variety of others(small bowel, ureter, renal) -2-5% of colon cancer cases -CRC penetrance=80% -Diagnosis criteria: •Amsterdam II •revised Bethesda
question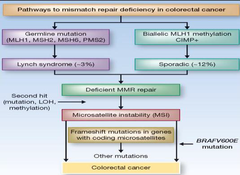
Pathways to mistmatch repair deficiency in CRC

answer
-all based on deficient MMR repair -MSI and frameshift mutations -2 molecular pathways that can lead to CRCs with MSI: 1-germline mutations in MMR gene followed by a second hit to the w/t copy that may occur due to a point mutation, LOH, or methylation •Germline MMR mutations→Lynch syndrome→accounts for 1/5 of all MSI CRCs 2-nonfamilial for of MSI: •due to epigenetic activation of MLH1 occuring in the background of hypermethylation of the promoters of multiple genes i.e. CIMP •sporadic tumors show CIMP and BRAFV006E hotspot mutations that serve to distinguish them from Lynch syndrome cancers
question
Mismatch repair(MMR) genes
answer
-behave like TSGs and critically maintain genomic stability -involved in DNA repair of incorrect pairing of bases -MMR mutations(>500 characterised) result in MSI
question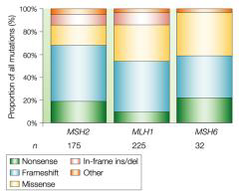
Germline mutations in MMR genes and HNPCC

answer
-MLH~50% -MSH2~40% -MSH6~10% -PMS2 Types of mutation: -Deletion -Loss of heterozygosity -Promoter methylation
question
Germline and somatic ε and δ mutations define a new class of hypermutated CRCs
answer
-identified rare syndrome based on mutations -Pol ε and δ replicate eukaryotic DNA →accuracy requires the polymerases' exonuclease(proofreading) domains -germline exonuclease domain mutations (EDMs) of POLE and POLD1 confer a high risk of multiple colorectal adenomas and CRC •problems with polymerases' exonuclease proofreading domains POLE and POLD1→higher risk of multiple colorectal adenomas -mutations associated with high penetrance, dominant inheritance but variable phenotype=polymerase proofreading-associated polyposis(PPAP) -somatic POLE EDMs are found in sporadic CRCs→few POLD1 EDMs detected -germline and somatic DNA polymerase EDMs→ultramutated, microsatellite-stable cancer(>1 million base substitutions/tumour)
question
Common disease-common/rare variant models
answer
-Mendelian inherited syndromes only explain a minor part of familial clustering of common cancers e.g. CRC -increased inherited risk of cancer involves genes of low-or moderate-penetrance→may have been difficult to identify -'common disease-common variant' model of predisposition: • alleles of high frequency (>10%) and low penetrance (< 2x increased lifetime risk) contribute to susceptibility to common human diseases -common disease-rare variant model: -both models have merits •require substantial sample sizes and latest technology→next generation sequencing
question
Genome wide association studies(GWAS)
answer
-common risk alleles for cancer are found by GWAS studies that used tagged SNPs •but identification of the disease-causing variants is challenging
question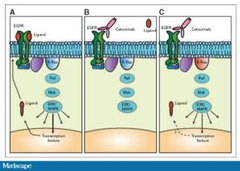
Genetics and CRC targeted therapy

answer
-targeted cancer therapies interfere with specific molecules involved in tumour growth and progression -therapies approved for metastatic CRC: •Cetuximab+ Panitumumab→bind to external portion of EGFR→prevent cellular proliferative/growth signalling >effective as monotherapy only in 10%-20% of mCRCs •Bevacizumab→binds VEGF→prevents new blood vessel growth(angiogenesis) -resistance can be caused by mutation in KRAS/BRAF •need to genotype CRCs for KRAS/BRAF mutations
question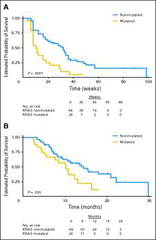
Biomarker of Centuximab Response in CRC

answer
-a biomarker was made to see who responded to centuximab and who doesn't -Mutations: •KRAS~40% of CRCs •BRAF~5-30% of CRCs -w/t KRAS tumours that respond initially almost always develop resistance within several months -38% of patients with w/t KRAS tumours developed KRAS mutations in their sera between 5-6mnths after treatment →mutations were present b4 treatment •few cells had the mutation and where selected for
question
Summary
answer
-CRC=colorectal/bowel cancer -abnormal growth of cells that can acquire the ability to invade/spread to other organs especially liver=metastatic -signs and symptoms=blood in stool, change in bowel movements, weight loss and fatigue -inflammatory bowel disease(Crohn's and UC) can increase risk of CRC -inherited disorders e.g. FAP and lynch can cause CRC→>5% of cases -CRC starts as a benign tumour(polyp, adenoma) that becomes cancerous over time -removal of benign tumour or resection of earliest stage →often =cure
question
Why is screening effective for early detection/prevention?
answer
-screening works by detecting polyps or early stage cancers followed by removal of abnormality -regular screening for and removal of polyps reduces risk of developing CRC-90% with colonoscopy -early detection of cancers that are already present in the colon increases the chance of successful treatment and decreases chances of dying as a result of the cancer
question
What are the protocols, advantages and short-comings of FOBT, flexible sigmoidoscopy and colonoscopy?
answer
-FOBT: lab test used to check stool samples for hidden blood→may indicate colon caner/polyps in the colon/rectum +ve: •no cleansing of colon necessary •samples collected at home •low cost compared to others •does not cause bleeding or tearing/perforation of the lining of the colon •24% sensitive -ve: •does not detect some polyps and cancers •dietary restrictions and changes e.g. avoiding meat for many days b4 test •false negatives and false positives can occur -flexible sigmoidoscopy: using a narrow tube of light with camera at end(sigmoidoscope) to examine the rectum and the lower/sigmoid colon. +ve: • quick and with few complications •discomfort is minimal •in some cases the doctor can perform a biopsy(take a piece of tissue) and/or remove polyps during the test •less extensive cleansing is required -ve: •doctor can only see the rectum and the lower part of the colon •polyps in upper part of colon are missed •small risk of bleeding or tearing/perforation of lining of the colon •additional procedures may be necessary -colonoscopy: •test that allows the doctor to look at the inner lining of the large intestine using a thin, flexible tube called the colonoscope +ve: •allows doctor to see the rectum and entire colon •doctor can perform a biopsy and remove polyps or other abnormal tissue during the test •most sensitive test available -ve: •may not detect all small polyps, non-polypoid lesions and cancers •thorough cleansing is necessary •some form of sedation is used •complications e.g. bleeding and or tearing of the lining of the colon can occur >serious complications are rare
question
is genetic screening and profiling of germline tumours and using next generation sequencing the way forward?
answer
-genetic testing for patients at high-risk of germline mutations e.g. MSI in lynch syndrome→targeted surveillance for CRC that extends to at risk-family members -genetic biomarkers that predict response to treatment are beginning to come into routine practice in the management of CRC e.g. KRAS mutational testing
question
what role might exercise play as a second therapy in CRC?
answer
-physical activity(PA) would counter obesity and obesity related health risks e.g. cancer -proposed mechanisms by which PA may lead to beneficial effects: •decreased intestinal transmit time •immune-up regulation •suppression of insulin and insulin like growth factor(IGF-1) mediated growth •most evidence comes from observational studies: significant benefit of 27% with PA irrespective of location of CRC •animal models of CRC has shown that exercise significantly reduces colon cancer incidence •PA is associated with physical fitness and possibly decreased all cause mortality •effect on quility of life and fatigue is unclear
question
what evidence is there for a diet high in red, processed meat, but low in fiber increasing CRC risk?
answer
-high red, processed meat diet: • eating lots of red/processed meat increases risk of bowel cancer •1/4 of bowel cancer cases in men and 1/6 in women are linked to eating red or processed meat •bowel cancer risk increases by 28% for every 120g of red meat eaten/day -low fibre diet: •recent study found more than 1/10 (12%) bowel cancers are linked to a low fibre diet •review of all studies on this topic has shown eating 10g fibre/day can reduce risk of bowel cancer by 10%
question
What evidence is there that aspirin and other non-steroidal anti-inflammatory(NSAIDs) drugs decrease CRC risk?
answer
-aspirin and NSAIDs: •shown to reduce incidence and mortality of CRC •a pooled meta-analysis of 8 RCTs with a total of 25,750 patients showed a 59% decrease in CRC mortality beginning 5yrs after aspirin treatment •shown to decrease incidence of any colorectal adenoma → •reduces risk of CRC in individuals who are carriers of HNPCC→by 59% -believed to exert their beneficial effect by inhibition of arachidonic acid metabolism through cyclooxgenase/prostaglandin H synthase



