Reactions and Reagent Structures of Chp. 16 – Flashcards
Unlock all answers in this set
Unlock answersquestion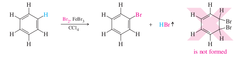
Important: * IDENTIFICATION method; causes decolorization, but SLOWLY * SUBSTITUTION not addition * all THREE double bonds are retained * only works with BROMINE

answer
benzene + bromine + carbon tetracholoride + ferric bromide --> hyrobromic acid (gas) + bromobenzene
question
Important: * NO decolorization (red to clear)
answer
benzene + bromine + carbon tetrachloride --> no reaction
question
Important: * NO decolorization (purple to brown)
answer
benzene + potassium permanganate + water --> no reaction
question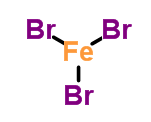
Name the compound (it has two names).
answer
ferric bromide iron (III) bromide
question
Important: * requires MORE ACTIVE CATALYST than just Pt, Pd, etc. * requires HIGHER PRESSURES of H2 * ?H < ?Hº of conjugated DIENE; resonance energy/double bond is amazingly low
answer
catalytic hydrogenation of benzene benzene + 3H2 --> cyclohexane
question
Important: * occurs by extremely FAST DIELS-ALDER reaction * reason why cyclobutadiene has NEVER been isolated and purified

answer
dimerization of cyclobutadiene monomer
question
Name the annulene.

answer
[4]annulene cyclobutadiene
question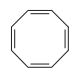
Name the annulene compound.

answer
[8]annulene cyclooctatetraene
question
Name the annulene shown in the picture.
answer
[10]annulene cyclodecapentaene
question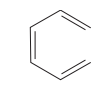
Name the aliphatic polyene.

answer
hexa-1,3,5-triene
question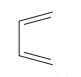
Name the aliphatic polyene shown in the picture.

answer
buta-1,3-diene
question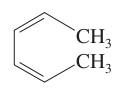
Draw and name the acyclic counterpart of cyclohexa-1,3-diene.

answer
cis,cis-hexa-2,4-diene
question
Give BOTH names of the cyclic polyene shown in its two most common conformations.
answer
cyclotetradecaheptaene [14]annulene LEFT: antiaromatic RIGHT: aromatic
question
Name the polycyclic compound.
answer
pyrene
question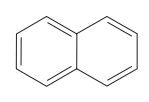
Name the aromatic polycylic compound.

answer
napthalene (C10H8) IMPORTANT: * simplest poylnuclear aromatic hydrocarbon (PAH or PNA) * lower resonance energy than benzene * 10 electrons in resonance system * has three Kekulé structures * MOTHBALLS
question
Give BOTH names of the cyclic polyene shown in four of its conformations.
answer
cyclodecapentaene [10]annulene
question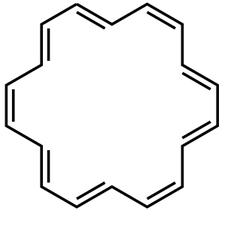
Give two names for the compound shown.

answer
cyclooctadecanonaene [18]annulene
question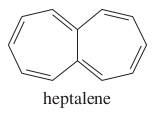
Give the name of the molecule.

answer
heptalene
question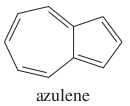
Give the name of the molecule in the image.

answer
azulene (an constitutional isomer of naphthalene)
question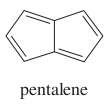
Give the name of the compound in the image.

answer
pentalene
question
IMPORTANT: * cyclopentadiene has a pKa of 16, near that of water! * must use a really strong base (hydroxide ion wouldn't deprotonate because water is a stronger acid) * note how the cyclopentadienyl anion can be written in shorthand
answer
cyclopentadiene + tert-butoxide ion --> tert-butanol + cyclopentadienyl anion (aromatic)
question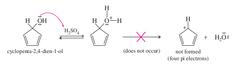
IMPORTANT: * the cyclopenta-2,4-dien-1-ol cation is too unstable for the hydronium to act as a leaving group because the ion is antiaromatic

answer
cyclopenta-2,4-dien-1-ol + conc. H2SO4 --> protonated cyclopenta-2,4-dien-1-ol [FINAL PRODUCT]
question
Name the cation; it has two names.
answer
tropylium ion cycloheptatrienyl cation
question
IMPORTANT: * the tropylium ion is a more stable base than the hydroxide ion
answer
cycloheptatrienol + aqueous solution of pH tropylium ion
question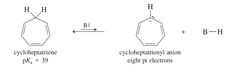
IMPORTANT: * sp3 hydrogen taken * reaction not favored * barely more acidic than propene * gains only one electron

answer
cycloheptatriene + very strong base --> cycloheptatrienyl anion + B-H
question
IMPORTANT: * forms a hydrocarbon dianion (rare) * cyclooctatetraene gains TWO electrons via the reaction * the hydrogens leave and do not retain any electrons * potassium donates two electrons to the hydrogen cations
answer
cyclooctatetraene + 2 potassium metal --> cyclooctatetranyl DIANION + 2K+ + H2 (gas)
question
Draw the structure of pyridine.
answer
IMPORTANT: * aromatic * pKb = 8.8 * usually undergoes substitution rather than addition
question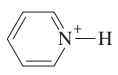
Draw the pyridinium ion.
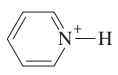
answer
IMPORTANT: * It is still aromatic. * pKa = 5.2
question
Draw the structure of pyrrole
answer
* pKb = 13.6 * strong acid usually protonates at C2 * when protonated at nitrogen, denoted "N-protonated pyrrole" * N-protonated pyrrole pKa = 0.4
question
IMPORTANT: * when imidazole becomes protonated, the nitrogens become chemically equivalent (isoelectronic) due to resonance
answer
imidazole + acid --> protonated imidazole [resonance] equilibrium
question
Draw the structure of purine.
answer
IMPORTANT: * four nitrogens total * three basic nitrogens * one nonbasic nitrogen * imidazole ring and pyrimidine ring fused together
question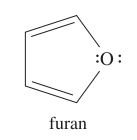
Draw the structure of furan.

answer
IMPORTANT: * once it's hydrogenated, it's known as tetrahydrofuran * the oxygen atom is sp2 hybridized, with one lone pair in a 2p orbital and one lone pair in an sp2 orbital * aromatic
question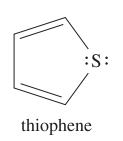
Draw the structure of thiophene.

answer
IMPORTANT: * also known as THIOFURAN * sulfur is sp2 hybridized with one lone pair in a 3p orbital and the other lone pair in an sp2 orbital * aromatic
question
Give both names of the compound shown.
answer
ammonia azane
question
Name the PAH.
answer
anthracene (C14H10) IMPORTANT: * can undergo addition reactions * 4 resonance structures
question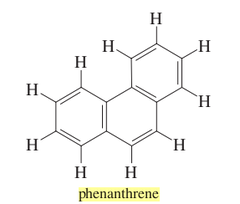
Name the PNA.

answer
phenanthrene (C14H10) * can undergo addition reactions * 5 resonance structures
question
IMPORTANT: * goes by 1,4-addition to maintain integrity of two isolated, aromatic benzene rings
answer
anthracene + Br2 in carbon tetrachloride (nonpolar solvent) --> 9,10-dibromo compound
question
IMPORTANT: * goes by 1,2-addition to maintain integrity of two isolated, aromatic benzene rings
answer
phenanthrene + Br2 in carbon tetrachloride (nonpolar solvent) --> 9,10-dibromo compound
question
Name the large polynuclear aromatic hydrocarbon.
answer
benzo[a]pyrene IMPORTANT: * one of the most thoroughly studied carcinogens * found in cigarette smoke * formed whenever organic compounds undergo incomplete combustion * epoxides form and react with DNA
question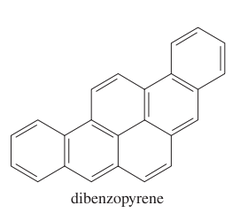
Name the six-ringed polynuclear aromatic hydrocarbon.

answer
dibenzopyrene
question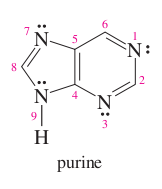
pyrimidine + imidazole =

answer
purine
question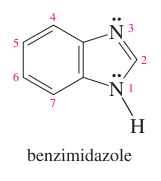
benzene + imidazole =

answer
benzimidazole
question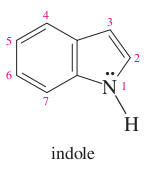
benzene + pyrrole =

answer
indole
question
benzene + pyridine =
answer
quinoline
question
benzene + furan =
answer
benzofuran
question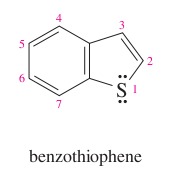
benzene + thiophene =

answer
benzothiophene
question
Please name the compound; give both its common name and IUPAC name.
answer
IMPORTANT: it's found in licorice
question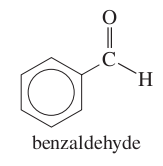
Name the compound; give both its common name and IUPAC name.

answer
IMPORTANT: it's found in Maraschino cherries
question
Give three names for the compound.
answer
propanone, dimethyl ketone, and acetone
question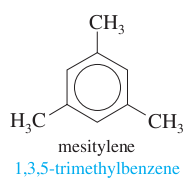
three methyls + benzene

answer
mesitylene 1,3,5-trimethylbenzene
question
two methyls + benzene; meta positioning
answer
m-xylene 1,3-dimethylbenzene
question
one methyl + one hydroxy group + benzene; para positioning
answer
p-cresol 4-methylphenol
question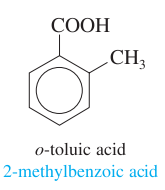
toluene + acetyl group; ortho positioning

answer
o-toluic acid 2-methylbenzoic acid
question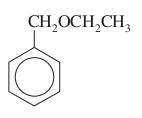
Give four names for the compound, tricky little bastard.

answer
benzyl ethyl ether (ethoxymethyl)benzene ?-ethoxytoluene benzoxyethane



