Nuclear Chemistry, Atoms: The Building Blocks of Matter – Flashcards
Unlock all answers in this set
Unlock answersquestion
The change in the identity of an isotope due to a change in the number of its protons
answer
Transmutation
question
When an isotope loses a particle with a 2 proton and a mass of 4
answer
Alpha Decay definition
question
When an isotope gains a proton through the loss of an electric charge on one of its neutrons
answer
Beta Decay definition
question
When an isotope decays to release only energy, not a particle
answer
Gamma Decay definition
question
a positive particle with the same mass as an electron given off as a result of a proton changing to a neutron
answer
Positron
question
the amount of time it takes for half of a substance to decay into another substance
answer
Half-life
question
a negative sub atomic particle
answer
Electron
question
the splitting of one large nucleus to create two smaller nuclei (two new elements with some mass being converted into energy)
answer
Fission
question
the combining of two smaller nuclei to create a larger nucleus (some mass is converted to energy)
answer
Fusion
question
the process using Carbon-14 to date materials that were once alive
answer
Carbon Dating
question
a charged atom
answer
Ion
question
an atom with fewer or more neutrons than the average form of that element
answer
isotope
question
"indivisible" (Greek meaning), the smallest particle of an element that retains the chemical properties of that element
answer
Atom
question
All matter is made of atoms and atoms are indivisible, cannot be subdivided, created or destroyed
answer
Dalton's Atomic Postulate 1
question
All atoms of the same elements are identical in mass and properties (the same)
answer
Dalton's Atomic Postulate 2
question
Compounds are combinations in whole number ratios of two or more types of atoms
answer
Dalton's Atomic Postulate 3
question
In chemical reactions, atoms are combined, separated or rearranged

answer
Dalton's Atomic Postulate 4
question
Compounds are made in definite proportions
answer
Dalton's Atomic Postulate 5
question
(Law of Lavoisier) in chemical reactions, the total mass is conserved
answer
Law of Conservation of Mass
question
(Proust) when a chemical compound is formed, their is a definite proportion of the atoms forming the compound (example: in table salt; sodium chloride; it ALWAYS has by mass, the same amount of sodium (Na , 39.34%) and chlorine (Cl, 60.66%)
answer
Law of Definite Proportion
question
if 2 or more different compounds are composed of the same 2 elements, then the ratio of the masses of the second element combined with a certain mass of the first element is always a ration of small whole numbers. (carbon and oxygen form carbon dioxide and carbon monoxide; the ratio of carbon to oxygen between dioxide to monoxide is always 2:1)
answer
Law of Multiple Proportions
question
number of protons of each atom of that element

answer
Atomic Number
question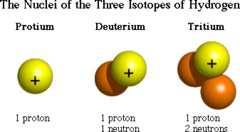
atoms of the same element that have different numbers of neutrons in the nucleus (so different atomic masses) Tin has the most isotopes (10); although they differ in masses isotopes do not differ significantly in their chemical behavior

answer
Isotope
question
the total number of protons plus neutrons that make up nucleus of an isotope
answer
Mass number
question
general term for a specific isotope of an element
answer
Nuclide
question
(amu) a unit of mass that describes the mass of an atom or molecule
answer
Atomic Mass Unit
question
(mol) the SI unit for amount of substance: the amount of a substance that contains as many particles as there are atoms in exactly 12g of carbon-12
answer
Mole
question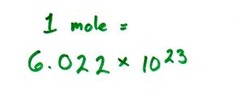
the number of particles in exactly one mole of a pure substance (6.022 1415 x 10^23 or rounded to 6.022 x 10^23 = exactly what 12g of carbon-12 atoms contains)
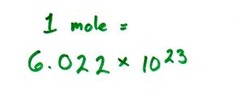
answer
Avogadro's number
question
equal to the atomic mass of the element; the mass of one mole of a pure substance is called the molar mass of that substance; written in units of g/mol; FOUND ON THE PERIODIC TABLE FOR EACH ELEMENT
answer
Molar mass
question
what make up an atom; the three main are protons,neutrons, and electrons
answer
Subatomic particles
question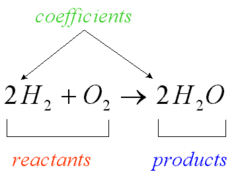
transformation of a substance or substances into one or more new substances
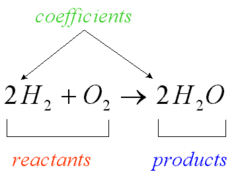
answer
Chemical Reaction
question
a very small region located at the center of an atom
answer
Nucleus
question
positively charged (+) particle equal in magnitude to the negative charge of an electron; at least one in every atom
answer
Proton
question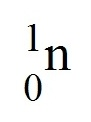
neutral particle; at least one or two in every atom
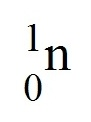
answer
Neutron
question
negative charged particle (-); surrounds the nucleus in an occupied region

answer
Electron
question
glass tubes; in late 1800s, experiments were performed where electric current was passed through various gases at low temp; the resulting glowing stream was called cathode rays
answer
Cathode-ray tubes
question
weighted average of the atomic masses of the naturally occurring isotopes of an element
answer
Average Atomic Mass
question
(1897) discovered electrons, charge to mass ratio and that all cathode rays are composed of identical negatively charged particles named electrons
answer
JJ Thomson
question
(1911) discovered the nucleus, protons; Gold foil experiment
answer
Rutherford
question
(1934) discovered the neutrons
answer
Chadwick.
question
the short-range proton-neutron, proton-proton, and neutron-neutron forces hold the nuclear particles together
answer
Nuclear forces
question
Mass # - atomic # = # neutrons (protons+neutrons)-protons=neutrons
answer
Neutron equation (find # of neutrons)
question
streams of negatively charged particles (electrons).
answer
cathode rays
question
JJ Thomson; negative electrons are spread evenly through the positive charge of the rest of the atom (think watermelon)
answer
plum pudding model (Thomson)
question
1. since atoms are electronically neutral they must contain a positive charge to balance the negative electrons 2. because electrons have so much less mass than atoms, atoms must contain other particles that account for most of their mass
answer
Robert A. Millikan (1909)
question
bombarded a thin piece of gold foil with a narrow beam of alpha particle, some particles were deflected back to source - reasoned this was the nucleus and it was a very small part of atom because the rest of the particles passed through undisturbed
answer
1911 - Rutherford, Geiger and Marsden
question
different elements differ in number of protons (positive charge) so the number of protons determines the atoms identity
answer
Nuclei
question
the short range proton-neutron; proton-proton and neutron-neutron forces that hold the nuclear particles together
answer
nuclear forces
question
Radius of an atom from the center of the nucleus to the outer portion of the electron cloud ; 1 pm = 10^-12(ten to the negative 12th power) meters or 10^-10 centimeters

answer
picometer (pm)
question
number of protons in each atom of that element

answer
atomic number
question
Identified by specifying their mass number in 2 ways: 1. Hyphen notation: hydrogen - 3 2. Nuclear symbol: 3 H 1 with mass number (neutron + protons) on top and atomic number (protons) on bottom
answer
Designating isotopes
question
all atoms compared to carbon-12 atoms (12 amu) so 1 amu = 1/12 of carbon
answer
atomic mass units (amu)
question
multiply mass of each type by the decimal fraction representing its % in the mixture 25% = .25 each weighing 2g; 75%=.75 each weighing 3g so (2g*.25)+(3gx.75)=2.75g
answer
calculating average atomic mass
question
(moles of substance) x molar mass = grams and (moles)(gram/moles)=grams

answer
gram/mole conversions
question
1911 model of the atom. small nucleus surrounded by electrons in orbit around it

answer
Rutherford model
question
Suggest that electrons move in a definite path around the nucleus. 1913
answer
Bohr atomic model
question
a mathematical model and most accurate model of an atom used today
answer
Quantum mechanical model (electron cloud model)
question
The first major model of the atom, developed in 1800.
answer
Dalton model
question
Basically, this model was a philosophical idea that everything could be broken down into one fundamental unit -- the atom. It could not be tested at that point in time because the Greeks did not posses the technology to observe anything that was so microscopic such as the atom.
answer
Greek model
question
the protons and neutrons in atomic nuclei
answer
nucleons
question
the reference to an atom in nuclear chemistry; identified by the number of protons and neutrons in its nucleus
answer
nuclide
question
difference between the mass of an atom and the sum of the masses of its protons, neutrons and electrons
answer
mass defect
question
conversion of mass to energy upon formation of the nucleus
answer
what causes mass defect?
question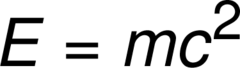
the energy release when a nucleus is formed from nucleons; E=mc^2
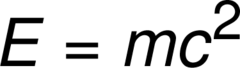
answer
nuclear binding energy
question
is the binding energy per of the nucleus divided by the number of nucleons it contains - the higher the binding energy the more tightly the nucleons are held together and are therefore more stable
answer
binding energy per nucleon
question
stable nuclei cluster over a range of neutron-proton ratios (the graph of elements and their isotopes plotted)
answer
band of stability
question
nucleons exist in different energy levels or shells in the nucleus
answer
nuclear shell model
question
Numbers of nucleons that represent completed nuclear energy levels: 2, 8, 20, 28, 50, 82, and 126 - the most stable nuclides
answer
magic numbers
question
reaction that affects the nucleus of an atom
answer
nuclear reaction
question
change in the identity of a nucleus as a result of a change in the number of its protons
answer
transmutation
question
the spontaneous disintegration of a nucleus into a slightly lighter nucleus, accompanied by emission of particles, electromagnetic radiation or both
answer
radioactive decay
question
1896 wrapped a photographic plate in lightproof covering and place uranium on top of it. Figured out didn't need sunlight to expose the plate, it was the radioactive decay of uranium that exposed the plate
answer
Henri Becquerel
question
particles or electromagnetic radiation emitted from the nucleus during radioactive decay
answer
nuclear radiation
question
an unstable nucleus that undergoes radioactive decay - all nuclides beyond atomic #83 are unstable and radioactive
answer
radioactive nuclide
question
two protons and two neutrons bound together and emitted from the nucleus during some kinds of radioactive decay; charge is 2+, represented by symbol
answer
alpha particle
question
an electron emitted from the nucleus during some kinds of ratioactive decay. atomic number 1+, mass stays the same
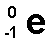
answer
beta particle
question
particle that has the same mass as an electron but has a postive charege, and is emitted from the nucleus during some kinds of ratio active decay
answer
positron
question
an inner orbital electron is captured by the nucleus of its own atom. the inner orbital electron combines with a proton and a neutron is formed; atomic number decreases by one but mass number doesn't change (stays the same)
answer
electron capture
question
high energy electromagnetic waves emitted from a nucleus as it changes from an excited state to a ground energy state
answer
gamma rays
question
alpha: your hand beta: aluminum gamma: lead neutrons: concrete
answer
penetration of particles
question
time required for half of the atoms of a radioactive nuclide to decay
answer
half life
question
a series of ratioactive nuclides produced by successive radioactive decay until a stable nuclide is reached
answer
decay series
question
heaviest nuclide of each decay series
answer
parent nuclide
question
nuclides produced by the decay of the parent nuclides
answer
daughter nuclides
question
radioactive nuclides not found naturally on earth
answer
artificial radioactive nuclides
question
bombardment of nuclei with charged and uncharged particles to make artificial radioactive nuclides; radioactive isotopes of all the natural elements have been produced this way
answer
artificial transmutations
question
elements with more than 92 protons in their nuclei - all are radioactive
answer
transuranium elements
question
unit used to measure nuclear radiation exposure
answer
roentgen (R)
question
unit used to measure the dose of any type of ionizing radiation that factors in the effect that the radiation has on human tissue
answer
rem
question
use exposure of film to measure the approximate radiation exposure of people working with radiation
answer
film badges
question
are instruments that detect radiation by counting electric pulses carried by gas ionized by radiation; typically used to detect beta particles, x rays and gamma radiation
answer
Geiger-Muller counters
question
instruments that convert scintillating light to an electrical signal for detecting radiation
answer
scintillation counters
question
process by which the approximate age of an object is determined based on the amount of certain radioactive nuclides present: Carbon 14 is radioactive has half life of approximately 5715 years can be used to estimate the age of organic material to about 50,000 years
answer
radioactive dating
question
radioactive atoms that are incorporated into substances so that movement of the substances can be followed by radiation detectors - used to diagnose cancer etc
answer
radioactive tracers
question
nucleus of very heavy atom (uranium) split into 2 or more lighter nuclei; releases high amounts of energy
answer
nuclear fission
question
reaction in which the material that starts the reaction is also one of the products and can start another reaction
answer
chain reaction
question
minimum amount of nuclide that provides the number of neutrons needed to sustain a chain reaction
answer
critical mass
question
use controlled fission chain reactions to produce energy and radioactive nuclides
answer
nuclear reactor
question
use energy as heat from nuclear reactors to produce electrical energy
answer
nuclear power plants
question
radiation absorbing material that is used to decrease exposure to radiation especially gamma rays from nuclear reactors
answer
shielding
question
neutron absorbing rods that help control the reaction by limiting the number of free neutrons
answer
control rods
question
used to slow down the fast neutrons produced by fission
answer
moderator
question
low mass nuclei combine to form a heavier more stable nucleus; opposite of fission; creates even more energy; cannot currently be controlled due to heat; named and explained by Lise Meitner
answer
nuclear fusion



