Kaplan MCAT Organic Chemistry Chapter 2: Isomers – Flashcards
Unlock all answers in this set
Unlock answersquestion
Structural isomers
answer
also called constitutional isomers. Least similar of the isomers. The only thing they share is their molecular formula.
question
Stereoisomers
answer
have same molecular formula. Share the same atomic connectivity. Stereoisomers differ in how the atoms are arranged in space. Any isomer that is not a structural isomer falls under this category.
question
Two classes of stereoisomers
answer
Conformational and configurational
question
Conformational isomers
answer
differ in rotation around a single bond . Same molecule but at a different place in their free rotation.
question
Anti-staggered conformation in Newman projection
answer
the two largest groups are antiperiplanar, in the same plane but on opposite sides. lowest energy - preferred
question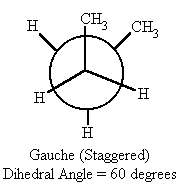
Gauche comformation

answer
two largest substituents are 60 degrees apart
question
Eclipsed conformation
answer
two largest substituents are 120 degrees apart
question
Totally eclipsed conformation
answer
largest substituents are 0˚ apart
question
What happens in a chair flip?
answer
all axial groups become equitorial and all equitorial groups become axial. All dashes remain dashes and all wedges remain wedges large, bulky groups prefer equatorial position
question
What position in a chair do bulky groups favor?
answer
equitorial positions
question
Configurational Isomers
answer
can only change from one form to another by breaking and reforming bonds. Two categories: Enantiomers and Diasteriomers
question
Enantiomers
answer
configurational isomers that are nonsuperimposable mirror images of one another. Have opposite configurations at every chiral center. have same chemical and physical properties different optical activity and reactions in chiral environments
question
Diasteriomers
answer
configurational isomers that are are not mirror images of each other. Differ at some but not all chiral centers for configuration. different chemical and physical prope
question
Racemic mixture
answer
when both enantiomers are present in equal concentrations, the rotations cancel each other out and no optical activity is observed
question
Priority in regards to E/Z
answer
higher atomic number means higher priority. If atomic numbers are equal, priority is determined by the next atoms outward.
question
Describe a fischer-projection
answer
horizontal lines indicate bonds that project outwards (wedges) and vertical lines inidicate bonds going into the plane (dashes)
question
angle strain
answer
bond angles deviate from their ideal values by being stretched or compressed
question
torsional strain
answer
cyclic molecules must assume conformations that have eclipsed or gauche interactions
question
nonbonded strain (van der Waals repulsion)
answer
nonadjacent atoms or groups compete for same space
question
What is the most stable conformation for cyclohexane?
answer
chair
question
Chirality
answer
if its mirror image can't be superimposed lacks internal plan of symmetry
question
Number of possible stereoisomers
answer
for n chiral centers, there are 2^n possible stereoisomers
question
Physical properties
answer
no change in composition of matter ex: boiling point, melting point, solubility, odor, color, density
question
Chemical properties
answer
reactivity of molecule, resulting in change in composition usually because of functional groups
question
meso compounds
answer
molecule with chiral centers that has an internal plane of symmetry not optically active because there is a plane of symmetry



