Chromatography: Separation by Adsorption
Unlock all answers in this set
Unlock answersquestion
A separation technique in which a mobile phase carrying a mixture moves in contact with a selectively *adsorbent* stationary phase
answer
What is chromatography?
question
The separation of a mixture of components occurs as a result of selective adsorbance of the components of the mixture on a stationary phase while carried out by a mobile phase
answer
On which principle are all chromatographic separations based?
question
Use gloves, oil from skin can affect results
answer
What precaution should be taking in handling the chromatography paper and why?
question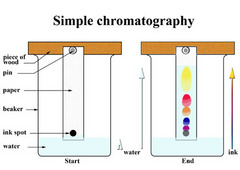
You may use paper clip and glass rod instead, pour water to depth of about 1cm if water soluble, use an organic solvent if they are 'water insoluble', mark a line on the paper with pencil of where the original sample of marker is put, making sure they are above solvent level

answer
Describe set-up of apparatus
question
Solvent rises up though paper, separation of colour
answer
What is seen as the apparatus is left alone?
question
The solvent is the mobile phase and as it moves up through the stationary phase (chromatography paper), the components of the mixture are adsorbed to a different extent on the paper
answer
Explain this separation of colours in the context of this experiment
question
Change the solvent/use a mixture of solvents, it will improve or worsen based on the nature of the solvent
answer
What do you change in the apparatus to show the effect of different mobile phases?
question
Which inks are mixtures of other inks and the inks they're a mixture of
answer
What can you tell about different inks after this experiment?
question
Universal indicator or chlorophyll or alcohol levels in blood
answer
Name other substances that can be analysed using chromatography
question
Apply mixture using dropper on paper about 2cm above solvent, place in tank with solvent, solvent moves up or down, state separation of components of mixture
answer
2008 q.2 (a) Describe experiment
question
Different absorbance on mobile and stationary phases
answer
2008 q.2 (b) Explain why the different components of the mixture travel different distances along the paper



