Biochemistry – Ch 14 – Metabolism – Flashcards
Unlock all answers in this set
Unlock answersquestion
Definition of Metaoblism
answer
It is the overal process through which living systems acquire and utilize free energy to carry out their functions Thye couple exergonic reactions of nutrient breakdown to endergonic processes required to maintain the living state
question
Catabolism
answer
(Degradation): Nutrients and cell constituents browken down to salvage componenets and/or generate energy.
question
Anabolism
answer
(Biosynthesis): Biomolecules are synthesized from simpler components
question
Autotrophs
answer
Self-feeders (synthesize thier own cellular constituents from H?O, CO?, NH?, H?S
question
Photoautotrophs
answer
Acquire free energy from sunlight
question
Chemolithotrophs
answer
Obtain free energy form oxidation of inorganic compounds such as NH?, H?S. Fe²?
question
Heterotorphs
answer
Oxidize organic compounds to make ATP
question
General on ATP
answer
ATP is the energy carrier for most biological reactions
question
Organisms can be classified by the identitiy of the oxidizing agent. (4)
answer
1. Obligate Aerobes 2. Anaerobes 3. Faculatative Anaerobes 4. Obligate Anaerobes
question
Obligate Aerobes
answer
Must use O2
question
Anaerobes
answer
Use sulfate or nitrate
question
Faculattive Anaerobes
answer
Can grow in presence or absence of O2 (i.e. E. coli)
question
Obligate Anaerobes
answer
Poisoned by O2
question
Metabolic pathyways are..
answer
Metabolic pathways are series of connected enzymatic reactions that produce specific products. There are over 2000 known metabolic reactions
question
Metabolites
answer
The reactants, intermediates and products of metabolic pathways.
question
Metabolic Reaction links
answer
Box 14-2
question
Carbohydrate Metabolism
answer
This figure shows most of the metabolic pathways that we will discuss in this half of the course, namely, the glycolysis pathway, gluconeogenesis, the citric acid cycle, and the pentose phosphate pathway. If you click on the glycolysis/ gluconeogenesis node, you will get the map on the next slide
question
Glycolysis/Gluconeogenesis
answer
• This figure shows the glycolysis and gluconeogenesis pathways. • It also give the enzyme classification (EC) code that will help you search for structures, sequences, and other information about it
question
Metabolic Pathways are comparmentalized.
answer
Pathways in eukaryotic cells occur in separate organelles or cellular locations. This exerts a greater control over opposing pathways and intermediates can be controlled by transport across the separating membraines
question
Oxidative Phosphorylation Occurs where?
answer
In the mitochondria
question
Glycolysis Occurs Where?
answer
Cytosol
question
Fatty acid Biosynthesis Occurs Where?
answer
Cytosol
question
Gluconeogenesis occurs where?
answer
In the liver to maintain constant level glucose in the circulation
question
Triacylglycerols are stored where?
answer
Adipose Tissues
question
Isozymes
answer
Enzymes that catalyze the same reaction but are encoded by different genes that have different kinetic of regulatory properties
question
Examples of Isozymes
answer
Lactate dehydrogenase (LDH): type M [skeletal muscle and liver] participates in the reduction of pyruvate to lactate (using NADH) while type H [heart muscle] catalyzes the reverse reaction.
question
Where is ATP made?
answer
In the mitochondria
question
Where is ATP used?
answer
In the cytosol
question
Where are fatty acids made?
answer
In the cytosol with the use of acetyl-CoA
question
Where is acetyl-CaA made?
answer
In the mitochondria
question
Major functions of the mitochondrion (5)
answer
1.TCA 2.ETC 3. oxidative phosphoylation 4. FA oxidation 5. amino acid breakdown
question
Major functions of the cytosol (4)
answer
1. Glycolysis 2. Pentose phosphate pathway 3. Fatty acid biosynthesis 4. Many rxns of gluconeogenesis
question
Major Functions of Nucleus (2)
answer
1. DNA replication and transcription 2. RNA processing
question
Major Function of the Lysosome (1)
answer
1. Enzymatic digestion of cell components and ingested matter.
question
Major Functions of the Golgi apparatus (2)
answer
1. Post translational processing of memraine and secretory proteins 2. Formation of plasma membrane and secretory vesicles
question
Major Functions of the RER (2)
answer
1. Synthesis of membrane-bound and secretory proteins
question
Major Function of the SER (1)
answer
Lipid and steroid biosynthesis
question
Major Function of the Peroxisome (glyoxysome in plants) (2)
answer
1. Oxidative reactions catalyzed by aa oxidases and catalase 2. Glyoxylate cycle reaction in plants
question
Roles of ATP and NADP+ in Metabolism
answer
In catabolic pathways, complex metabolites are exergonically broken down into simpler products, creating ATP or NADPH. In anabolic processes, simple molecules are converted inot complex molecules at the expense of degradation of the energy sotrage molecules, ATP and/or NADPH.
question
Very few metabolites are used to synthesize a large variety of biomolecules (3)
answer
• Acetyl-Coenzyme A (acetyl-CoA) • Pyruvate • Citrate cycle intermediates
question
Three main pathways for energy production
answer
• Glycolysis • Citric acid cycle • Oxidative-Phosphorylation
question
Overview of Catabolism (4)
answer
• Complex metabolites are broken down into their monomeric units • Then to the common intermediate, acetyl-CoA • The acetyl group is then oxidized to CO2 via the citric acid cycle while NAD+ and FAD are reduced to NADH and FADH2. • Reoxidation of NADH and FADH2 by O2 during oxidative phosphorylation yields H2O and ATP
question
Co2 is...
answer
The most oxidized state
question
Thermodynamic considerations
answer
A + B ? C + D; ?G = ?Go' + RT ln ([C][D]/[A][B]) When close to equilibrium, [C][D]/[A][B]?Keq and ?G ? 0. • This is true for many metabolic reactions - near-equilibrium reactions • When reactants are in excess, the reaction shifts toward products • When product are in excess, the reaction shifts toward reactants
question
However, some reactions are not near equilibrium and are thus irreversible
answer
- This is true of highly exergonic reactions - These metabolic reactions therefore control the flow of reactants through the pathway/cycle and they make pathways irreversible. 1. Metabolic pathways are irreversible 2. Every metabolic pathway has a first committed step 3. Catabolic and anabolic pathways must differ (so that they can be separately regulated)
question
Metabolic Pathways are irreversible
answer
They have large negative free energy changes to prevent them running at equilibrium. If two metabolites are interconvertible, the two interconversion pathways must be different Independent routes means independent control of rates. The need to control the amounts of either 1 or 2 independent of each other.
question
4 Types of control Flux at committed step(s)
answer
1. Allosteric control 2. Covelent modification 3. Substrate cycles 4. Genetic control
question
Allosteric Control
answer
By sustrates, products or coenzymes of the pathway (i.e. CTP in ATCase)
question
Covelent Modification
answer
(de) phosphorylation by (phosphatases) kinases which are themselves regulated.
question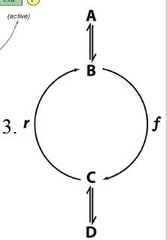
Substrate Cycles

answer
Fluxes through r and f can be separately regulated. One way exercgonic reaction (intermediates)
question
Genetic Control
answer
up or down regulated production or activation of an enzyme
question
Thermodynamics of phosphate compounds
answer
Adenosine diphosphate, one phosphoester bond and one phosphoanhydride bond Adenosine monophosphate one phosphoester bond. Which bonds are exergonic? High energy bonds. Hydrolysis of ATP
question
Coupling of Reactions
answer
These highly exergonic reactions are coupled to numerous endergonic biochemical processes so as to drive them to completion.ATP is generated by coupling its formation to more highly exergonic metabolic reactions The bioenergetic utility of phosphoryl-transfers stems from their kinetic stability to hydrolysis combined with their capacity to transmit relatively large amounts of free energy. ?G of ATP hydrolysis varies with pH, divalent metal ion concentration, and ionic strength
question
?G of phosphoenolpyruvate
answer
-61.9 kJmol?¹
question
?G of ATP
answer
-30.5 kJmol?¹
question
The P~P is a high energy bond
answer
Because of the concentrations of ATP, ADP, and Pi, the ?G of a reaction is usually -50 kJ/mol. Usually anything over 25 kJ/mol is called a high energy bond. These bonds are sometimes designated as a ~, or a squiggle: AR-P~P~P (adenyl, ribosyl, phosphoryl).
question
Why is the hydrolysis of ATP energetic?
answer
1. Resonance stabilization of a phosphoanhydride bond is less than that of its hydrolysis products. 2.Electrostatic repulsion between three of four negative charges on the phosphate at neutral pH.?G becomes even lower at higher pH values which produces more charge. 3.Solvation energy of a phosphoanhydride bond is less than that of its hydrolysis products.
question
Coupled Reactions
answer
The hydrolysis will be high nerg. The tautomerization will be very exergonic. Add together.
question
Resonance structures for phosphate bonds
answer
In phosphoanhydride, the P=O are each competing for the same anhydride oxygen lone pairs. In the separated phosphates, there is no competition so the resonance is better. Finally, there is electrostatic repulsion between adjacent O- atoms in the phospho- anhydride (see zigzag line). This repulsion leads to destabilization of this form, favoring hydrolysis. They repulse each other (trying to escape each other) now water can stabilize each other.
question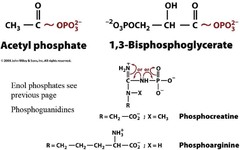
Other High Energy Compounds

answer
All have properties like ATp
question
Compounds liek ?-D-glucose-6-phosphat and 1-Glycerol-3-phosphat have...
answer
...smaller ?G's than ATP and have no significant resonance differences or charge repulsions
question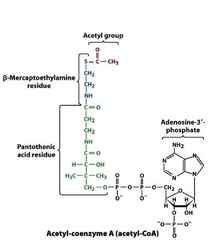
Thioesters***

answer
• Thioesters are found today in Coenzyme A (CoA) which links to various groups, most notably acetyl and is a common product of carbohydrate, fatty acid, and amino acid catabolism • Coenzyme A is sometimes written as CoASH since it has a reactive SH group • ?G0' for hydrolysis of the thio- ester bond is -31.5kJ/mol, 1kJ/mol more then ATP hydrolysis!!
question
The Role of ATP
answer
1. Kinases 2. Introconversion of nutcleoside triphosphates ATP, GTP, CTP, UTP 3. Physiological Processes 4. Additional phophoanhydride cleavage in highly endergonic reactions.
question
ATP as Kinases
answer
Early stages of nutrient breakdown transfers a phosphate to sugars
question
Formation of ATP
answer
1. Substrate level phosphorylation - direct transfer of a phosphate group to ADP from a high energy compound. 2. Oxidative phosphorylation and photophosphorylation- electron transfer generates an ion gradient that is used to generate ATP. 3. Adenylate kinase reaction AMP + ATP ? 2ADP ATP turnover per hour for the average person (about 3 moles) ATP + creatine ? phosphocreatine + ADP for ATP storage; ATP buffer in muscle and nerve cells.
question
Oxidation and Reductions
answer
Electron transfer reactions are of great biological importance
question
Oxidation and reductions: The mitochondrial electron transport chain
answer
In mitochondrial electron-transport chain, electrons are passed from NADH along a series of acceptors of increasing reduction potential (including FAD) until O2
question
Oxidation and reductions: ATP generation
answer
ATP is generated from ADP and Pi by coupling its synthesis to this free energy cascade.
question
Oxidation and reductions: NADH
answer
NADH thereby functions as an energy-rich electron transfer coenzyme
question
Oxidation and reductions: NADH and NAD+
answer
Oxidation of NADH to NAD+ supplies sufficient free energy to generate 3 ATPs.
question
Oxidation and reductions: NAD+
answer
NAD+ is an electron acceptor of many exergonic metabolite oxidations
question
Oxidation
answer
Loss of electorns
question
Reduction
answer
Gain of Electrons
question
Oxidation and reductions
answer
Many redox reactions involve the breaking of a C-H bond and the loss of two bonding electrons. Electron acceptors will lose electorns until it his state of CO2. NADH will provide extra electrons in mitochondria for ATP
question
Reduction of NAD+ to NADH
answer
NAD+/NADH and FAD/FADH2 are the main electron carriers • A hydride ion (H-) attacks NAD+ resulting in NADH, the reduced form of NAD+ • The final recipient of electrons is O2, however, O2 can only accept electrons one at a time • FAD can provide electrons one at a time
question
Reduction of FAD to FADH?
answer
Better for providing electrons • FAD accepts two hydrogen atoms (H•), one at a time resulting in FADH• And FADH2 • FAD is the oxidized form and FADH2 is the reduced form H2O at end of ETC
question
Experimental approaches to study metabolism (3)
answer
Metabolic pathways can be understood at several levels 1. Sequence of reactions by which a nutrient is converted to end products. 2. Mechanism by which a intermediate is turned into its successor. 3. Regulation of the flow of metabolites in a pathway.
question
Inhibitor and growth studies
answer
Inhibitors and growth studies are used to see what is blocked. If a reaction pathway is inhibited, products before the block increase and intermediates after the block decrease in concentration
question
Genetic defects cause intermediates to accumulate (alcaptunurea)
answer
Usually degradates into water and CO2. But causes phenylpyruvate to accumulate and hurts brain. So must minimize ingestion of compound to minimize accumulation of intermediates.. The last step of Phyenyalanine?Tyrosine?p-hydroxyphenylpyruvate?Homogentisate to H2O + Co2 doesn't work
question
ATP in PHysiological Processes
answer
Muscle contraction Transport of ions agains concentration gradiets
question
Radioactive tracers
answer
Can determine precursor in reactions and degradation time of precursor by seeing how long it took for it to get lableld. Example: heme. Label each possible precursor, will be radioactive when the right one is used



