solutions vocab
Unlock all answers in this set
Unlock answersquestion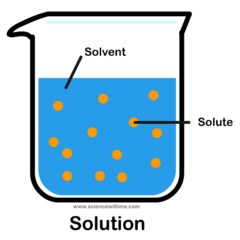
solution

answer
A mixture that forms when a solute is dissolved in a solvent. A solution is formed when salt and water mix together.
question
solute

answer
A substance that is dissolved in a solution. Salt is the solute when being mixed with water.
question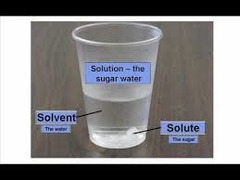
solvent

answer
The substance in which the solute dissolves in. The most commonly used solvent is water.
question
conductivity

answer
ability to conduct an electric charge; a measure of dissolved solids (mg/L). Acids and bases both have conductivity, so they can hold an electric charge.
question
concentration

answer
A measurement of how much solute exists within a certain volume of solvent. The more solute added to a substance, the higher the concentration is.
question
dissolving

answer
The process of mixing a solute in a solvent to produce a homogeneous mixture. An base is any compound forming hydroxide ions in dissolved water.
question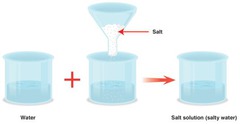
solubility

answer
A measure of how much solute can dissolve in a given solvent at a given temperature. The more solute being dissolved, the higher the solubility of the substances.
question
acids

answer
compounds that form hydrogen ions when dissolved in water. Lemons contain citric acid in it which causes lemons to taste so sour.
question
bases

answer
Substances that produce hydroxide ions when dissolved in water. Bases have a bitter taste and are slippery to touch.
question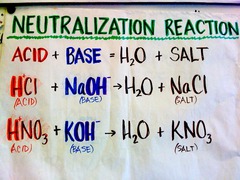
neutral

answer
A substance that is 7 on the pH scale. Water is neutral because it has a pH of exactly 7.
question
mixtures

answer
2 or more components physically mixed together; atoms and molecules don't change as there is no chemical bonding. Can separate out the components. Chex Mix is considered a mixture because the substances do not combine chemically and you could separate out the components.
question
pH scale

answer
A range of values used to express the concentration of hydrogen ions in a solution. Acids are to the left of 7 on the pH scale.
question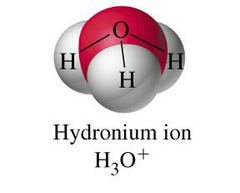
hydronium ions

answer
A positively charged ion formed when an acid dissolves in water. The symbol for a hydronium ion is H3O+.
question
hydroxide ions

answer
A negatively charged ion made of oxygen and hydrogen (OH-). Hydroxide ions are formed when bases dissolve in water.
question
litmus paper

answer
An example of an indicator that changes color in a solution and is used to tell if a solution is acidic or basic. If the red litmus paper turns blue, the substance is a base.
question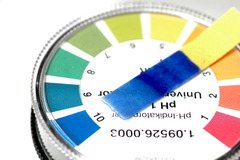
Indicator

answer
A compound that changes color in the presence of an acid or a base. Litmus paper is an example of an indicator because it color according to if the substance is an acid or base.
question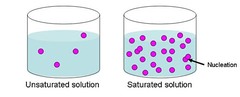
saturated

answer
Containing the highest amount of substance it can hold. Is NaCl saturated at 50 degrees at 70g in 100mL of water?
question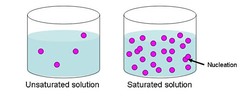
unsaturated

answer
A solution that contains less than the maximum amount of dissolved solute in a concentration. If the solution is unsaturated, it can still hold more dissolved solution.
question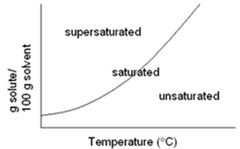
supersaturated

answer
A solution that holds more dissolved solute than is required to reach equilibrium at a given temperature. Not all the solute will dissolve if the solution is supersaturated.
question
solubility curve

answer
A graphic representation of the variation with changing temperature of the solubility of a given substance in a given solvent. Temperature will always be on the bottom of a solubility curve graph.