L7-Colorectal Cancer – Flashcards
Unlock all answers in this set
Unlock answersquestion
Colorectal Carcinoma
answer
ID risk factors for colon cancer Compare prevention and screening for ave risk and high risk subgroups Describe treatment options for colorectal cancers based on stage, patient-specific factors and treatment history Outline the indication and principles of chemotherapy for colon cancer from supporting literature List and describe the adverse effects of colorectal cancer chemotherapy that require patient counseling and specific monitoring Recommend the peri-chemotherapy agents to prevent or treat specific toxicities for the more common colorectal cancer regimens
question
Colorectal carcinomas
answer
Colon cancer - refers to group of adenocarcinomas that arise from musous and fluid secreting cells in the colon Rectal cancer - refers primarily to adenocarcinomas that occur between the anal dentate line to the sigmoid colon at the peritoneal reflection. A distance between 10 cm and 15 cm from the anal verge. Rare colon and rectal cancers: May both arise from carcinoid, lymphoma, neuroendocrine, and gastrointestinal stomal tumors
question
Epidemiology
answer
Estimated new cases and deaths from colon and rectal cancer in US in 2017 New cases: 95,520 (colon); 39,910 (rectal) Deaths: 50,260 (colon and rectal combined) 3rd most frequently diagnosed cancer in the US in both men and women 2nd leading cause of cancer death in men US 3rd leading cause of cancer death in women US
question
Epidemiology outlook
answer
Overall colorectal cancer morality has decreased slightly over the last 30 years most likely due to improved screening and better treatment modalities Occurs equally in men / women African-Americans have the highest colorectal cancer rates and morality
question
Epidemiology Breaking News 2

answer
Age specific CRC risk escalated back to levels of people from 1890 births - need to spread awareness
question
Epidemiology Risk
answer
Lifetime Risk - 1 in 21 men and 1 in 24 women will be diagnosed with cancer of the colon and rectum in life Over 1 million survivors in US 5 year relative survival Metastatic disease = 11.6% overall < 6% Localized disease = 90.4% Localized disease is highly treatable and often cured with surgery resulting in cure rate of approximately 50% of localized cases
question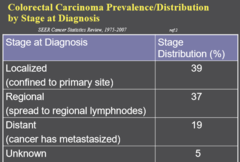
Colorectal Carcinoma Prevalence/Distribution by stage at diagnosis

answer
Localized - primary site - 39% Regional - spread to regional lymphnodes - 37% Distant - cancer metastasized - 19% Unknown - 5%
question
Risk Factors
answer
Age - Median age at diagnosis is 70 years -Heredity syndromes and genetic risk factors - (colorectal cancer) -1st degree family history of colorectal cancer - 5-10% of all cases -Ulcerative colitis or Chron's disease - personal history (colon cancer) -Ovarian, endometrial, or breast cancer - personal history (colorectal cancers)
question
Hereditary syndromes
answer
FAP - Familial Adenomatous Polyposis Autosomal dominant, APC gene mutation Hundred of adenomatous polyps after first decade Accounts for 1% of call cases Lynch Syndrom = HNPCC Hereditary Non-Polyposis Colorectal Cancer Autosomal dominant, not many polyps Accounts for 3-5% of all cases Mutations in DNA repair enzyme genes: MLH1, MSH2, MSH6, PMS1, PMS2 PDL-1 may work with MLH
question
Celecoxib and FAP
answer
Celecoxib is no longer indicated for treatment of adenomatous colorectal polyps due to the results of two 3-year RCTs which identified a dose related increase in the composite endpoint (CVDeath, MI, stroke) compared with placebo. Interesting because NSAID do the same things
question
Hereditary Syndromes
answer
Peutz-Jeghers MYH-associated neoplasia Juvenile polyposis
question
Absolute Risks of Colorectal Cancer for mutation carriers in Hereditary Colorectal Cancer Syndromes
answer
Syndrome - Absolute CRC risk in mutation carriers FAP - 90% by age 45 Attenuated FAP - 69% by age 80 Lynch - 40 - 80% by age 75
question
Risk Factors - Lifestyle-related
answer
Obesity Inactivity - sedentary life style Smoking Heavy Alcohol Use Diet - Red and processed meat and high fat low fiber diet
question
Risk Reduction / ChemoPrevention
answer
Weight loss Low fat diet, avoidance of red meat Exercise Reduction of heavy alcohol use Smoking cessation NSAIDS - growing preventative evidence Celecoxib - controversial studies
question
Signs and symptoms of colorectal cancer
answer
Change in bowel habits - diarrhea, constipation, narrowing of stools that last more than few days Tenesmus - sensation of the urgent need to defecate that is not relieved by doing so Rectal bleeding, dark stools, blood in stool (often, though, the stool will look normal) Cramping or abdominal pain Weakness and fatigue, anemia Unintended weight loss most sx commonly caused by other conditions such as infection, hemorrhoids and IBS
question
Colorectal Cancer Screening
answer
NCCN Average Risk Screening Criteria Age 50;= years No history of adenomas or colorectal cancer No history of inflammatory bowel disease Negative family history Screening schedule is dependent on method used
question
High Risk Screening Recommendations
answer
Basic High Risk Categories: History of polyps on prior colonscopy - intervals shorten from 5-10 years to months based on number and nature of polyps Colorectal Cancer or history of colorectal cancer surgery (monitoring) Family history - ranges from ; 1st degree relative to hereditary syndromes with more younger initial screening ages and more frequent screening
question
Colorectal Cancer Screening Guidelines NCCN
answer
NCCN recommended modalities/schedules Colonscopy ***-Current preferred screening method every 10 years (if available)*** -Required procedure for confirmation of positive findings from other tests Flexible sigmoidoscopy ***Must be repeated every 5 years*** with or without gFOBT or FIT = Fecal Occult Blood Test and ____ Double contrast barium Enema Patients unable colonscopy or with incomplete results ***Repeat every 5 years***
question
Fecal Occult Blood Test - gFOBT
answer
***Annually*** Stool guaiac based - dietary issues, many false negatives/positives colonscopy should be done if results positive
question
Fecal Immuno-histochemical testing - FIT
answer
***Annually*** Immuno-histochemical assay for human hemoglobin Positive if ; 100 mg of hemoglobin per mL of buffer Colonscopy should be done if test results are positive
question
CT Colonography (virtual colonscopy)
answer
***Every 5 Years***
question
Cologuard - Multi-Target DNA Stool Testing
answer
Stool based colon screening test that detects the presence of blood and altered DNA May indicate certain kinds of abnormal cells related to colon cancer or pre-cancerous growths Cells shed from the cells in the intestinal lining include abnormal cells Detects KRAS mutations, aberrant NDRG4 and BMP3 methylation, and beta-actin, plus hemoglobin immunoassay Results of these are fit onto a logistic regression analysis which generates a score
question
Cologuard - Multi-Target DNA Stool Testing 2
answer
Logisitic regression scores ; 183 indicating a positive results for CRC 2014 NEJM study = sensitivity for detecting colorectal cancer 92.3% w/ DNA testing vs 73.8 w/ FIT Cologuard detects cancer accurately 92% of time May maintain normal diet and medications for this test. Cologuard requires 1 bowel movement and addresses the entire GI tract Results mailed to MD in 2 weeks from receipt of sample by lab. ***Colonscopy needed if tests are positive*** Frequency is TBD but recommended at 3 years
question
CEA
answer
Carcinoembryonic antigen Oncofetal protein expressed during embryonic development 1st tumor marker ID-d in 1965 from human colon cancer tissue Glycoprotein - mw 180 kDa Increased in many gastrointestinal tumors Increased in non-malignant disease states such as pancreatitis, hepatitis, renal failure and amoking and therefore limits CEA screening potential
question
CEA Use
answer
Normal range = 0 - 5.0 ng/mL Often very elevated in both colon ; rectal cancer ****SHOULD NOT USED AS BE SCREENING TOOL Uses included detection of recurrence following surgery tumor response to chemo and or radiation disease progression if elevated initially
question
Colorectal Cacner Staging S1 and S2
answer
TNM Staging: T determined by depth of tissue invasion by tumor: Stage I: T1 - tumor invades submucosa T2 - turmor invades muscularis propia Stgae II: T3 - IIA invasion of the pericolorectal tissues T4a - IIB penetrates surface of visceral peritoneum T4b - IIC invasion or adherence to other organs or structures
question
Colorectal Cacner Staging S3 and S4
answer
Stage III: Positive regional nodes - A through C determined by number of nodes and depth of invasion Stage IV: Any T and any N IVA - metastasis confined to one organ IVB - metastases to more than one organ/site or peritoneal involvement
question
Work-up for non-metastatic diseases: Stage I, II, III
answer
Pathology review including lymph nodes Colonscopy CBC, platelets, chem profile, CEA Chest/abdominal/pelvic CT w/ contrast PET-CT scan is not routinely indicated
question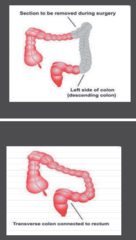
Colon Cancer Stage I Treatment

answer
Surgery Wide surgical resection and anastomosis via open colectomy with enbloc removal of at least 12 regional lymph nodes. Now sample up to 26. Laparoscopic assisted colectomy (LAC) may be performed if surgeon is certified in LAC and locally advanced disease, adhesions and rectal involvement have been ruled out Attached Transverse colon to rectum after remove of descending colon
question
Colon Cancer Stage II Treatment
answer
Surgery Wide surgical resection and anastomosis via open colectomy with enbloc removal of at least 12 regional lymph nodes Laparoscopic-assisted colectomy LAC may be performed if surgeon is certified in LAC and locally advanced disease, adhesions and rectal involvement have been ruled out
question
Colon Cancer Stage II Treatment Adjuvant Chemo
answer
Adjuvant Chemo Controversial: minimal evidence-based survival benefit Evidence is inconsistent tat adjuvant 5-fluorouracil 5-FU based chemo is associated with an improved overall survival OS compared to surgery alone MSI-H patients - good prognosis no 5FU adjuvant benefit - consider MMR testing (mis-match repair) Adjuvant chemo does not improve survival more than 5% NCCN revision removes FOLFOX as option for the treatment of SII colorectal cancer
question
Colon Cancer Stage III Treatment
answer
Surgery wide surgical resection and anastomosis via open colectomy with sampling of at least 12 regional lymph nodes and removal of all involved nodes Chemotherapy Adjuvant chemotherapy with ***mFOLFOX6*** as prefered regimen (NCCN Cat 1) Other options: CAPOX 5FU-Leucovorin single agent capecitabine
question
Colon Cancer Stage III Treatment Contd
answer
Patients where treatment with 5-FU leucovorin is planned, capecitabine is an equivalent alternative No role for irinotecan bevacizumab panitumumab cetuximab in stage II or III disease outside of trials
question
Work up metastatic disease Stages IV - any T, any N, M1
answer
Colonscopy CBC, platelets, chem profile CEA Chest/abdominal/pelvic CT ***w/ contrast*** KRAS, NRAS, BRAF, MSS gene status of tumor Needle biopsy if indicated PET-CT scan only if potentially surgically curable M1 Multidisciplinary team evaluation with experience in resection of hepatobiliary and lung metastases
question
Colon Cancer Stage IV Treatment 1
answer
Resectable synchronous liver and/or lung metastases: Surgery: Colectomy and resection of synchronous or staged liver and/or lung metastases Chemotherapy: Neoadjuvant chemo (2-3 months duration) for unsectable colon disease that may be reduced to resectuable status (not often) followed by synchronous or staged colectomy and/or staged resection of metastatic disease. (surgery before chemo) Adjuvant chemo after colectomy with chemotherapy for 2-3 months duration (surgery after chemo)
question
Colon Cancer Stage IV Treatment 2
answer
Resectable synchronous liver and/or lung metastases - neoadjuvant and adjuvant chemo: mFOLFOX6 +/- bevacizumab FOLFIRI +/- bevacizumab or +/- ziv-alfibercept CapeOX +/- bevacizumab or For KRAS WT (wild type) gene only mFOLFOX6 +/- cetuximab or panitumumab FOLFIRI +/- cetuximab or panitumumab
question
Colon Cancer Stage IV Treatment 3
answer
Unresectable synchronous liver and/or lung metastases or metanchronous metastases any site: Chemotherapy regimens: mFOLFOX6 +/- bevacizumab FOLFIRI +/- bevacizumab or +/- ziv-alfibercept CapeOX +/- bevacizumab or FOLFOXIRI for aggressive disease Surgery Colon resection only if imminent risk of obstruction or bleeding
question
Colon Cancer Stage IV Treatment 4
answer
Unresectable synchronous liver and/or lung metastases or metachronous metastases any site KRAS WT (wild type) gene only: Chemotherapy regimens: mFOLFOX6 +/- cetuximab or pantitumumab FOLFIRI +/- cetuximab or panitumumab
question
Colorectal Cancer Chemo Regimens 1
answer
Capecitabine (single agent) 5-FU / leucovorin CapeOX mFOLFOX6 FOLFIRI Stage IV only FOLFOXIRI Stage IV only +/- bevacizumab Stage IV only +/- ziv-alfibercept FOLFIRI ONLY +/- cetuximab or panitumumab (KRAS wild type only) Fegorafenib 4th line therapy only Trifluridine/tipiracil 4th line therapy only
question
Colorectal Cancer Chemo Regimens 5-FU / Leucovorin
answer
5-FU / Leucovorin Leucovorin 400mg/m2 IV over 2 hours on day 1, followed by: 5-FU bolus 400 mg/m2 and then 5-FU bolus 1200 mg/m2/day x 2 days (total 2400 mg/m2 over 46-48 hrs) continuous infusion repeat every 2 weeks
question
Colorectal Cancer Chemo Regimens CapeOX
answer
Oxaliplatin 130 mg/m2 IV day 1 Capecitabine 850-1000 mg/m2 BID for days 1-14 Repeat every 3 weeks (6 other variations of this regimen exist)
question
Colorectal Cancer Chemo Regimens mFOLFOX6 Workhorse for COLRECTAL CANCER!!
answer
Oxaliplatin 85mg/m2 over 2 hours on day 1 Leucovorin 400 mg/m2 IV over 2 hours on day 1 (may be infused with oxaliplatin - IV compatible) 5-FU 400mg/m2 IV bolus on day 1 after leucovorin then 5-FU 1200 mg/m2/day x 2 days total 2400 mg/m2 over 46-48 hours continuous infusion repeat every 2 weeks
question
Colorectal Cancer Chemo Regimens Capecitabine single agent
answer
1000 mg - 1250 mg/m2/dose PO BID x 14 days every 21 days
question
Colorectal Cancer Chemo Regimens FOLFIRI
answer
Stage IV ONLY Irinotecan 180 mg/m2 IV over 30-90 mins day 1 Leucovorin 400 mg/m2 IV to match duration of irinotecan infusion day 1 5-FU 400 mg/m2 IV bolus on day 1 (after leucovorin) 5-FU 1200 mg/m2/day x 2 days (total 2400 mg/m2 over 46-48 hours) continuous infusion Repeat every 2 weeks (1 cycle = 2 weeks)
question
Colorectal Cancer Chemo Regimens FOLFOXIRI
answer
Irinotecan 165 mg/m2 IV day 1 Oxaliplatin 85 mg/m2 over 2 hours on day 1 Leucovorin 400 mg/m2 IV over 2 hours on day 1 5-FU 3200 mg/m2 continuous infusion over 48 hours starting on day 1 repeat cycle every 2 weeks (1 cycle = 2 weeks)
question
Colorectal Cancer Chemo Regimens Bevacizumab + 5 FU containing regimens (FOLFOX or FOLFIRI)
answer
Bevacizumab 5 mg/kg IV every 2 weeks
question
Colorectal Cancer Chemo Regimens Cetuximab
answer
KRAS, NRAS wild type gene only Cetuximab 400 mg/m2 1st infusion then 250 mg/m2 IV weekly Cetuximab 500 mg/m2 IV every 2 weeks
question
Colorectal Cancer Chemo Regimens Panitumumab
answer
KRAS, NRAS wild type gene only Panitumumab 6 mg/kg IV over 60 mins every 2 weeks
question
Rectal Cancer Treatment Principles that differ from colon cancer
answer
surgical resection must consider focus on maintenance or restoration of normal anal sphincter, genitourinary and sexual function Radiation Therapy RT in combo w/ radio-sensitizing chemo limited fluoropyrymidines is the fundamental adjuvant treatment modality Other chemo agents are not used with RT Chemo therapy incorporated infusion 5-FU or capecitabine not to exceed 825 mg/m2 per dose for the duration of radiation treatment
question
Rectal Cancer Chemo Regimens
answer
Chemo and treatment principles same as colon cancer for neoadjuvant or advanced metastatic rectal cancer mFOLFOX6 CapeOX FOLFIRI +/-Bevacizumab +/-cetuximab or panitumumab if KRAS wild type
question
Agents indicated for Colorectal Carcinomas
answer
5-FU Admin: IV bolus after leucovorin and then continuous infusion in most regimens Toxicities Neutropenia is dose limiting w/ IV bolus Continuous infusion dose-limiting toxicities diarrhea and hand-foot syndrome Mucositis with both routes Less common toxicities Skin discoloration, nail changes, photosens, neurologic toxicity Rare: cardiac arrhythmias, ischemia, and cardiac myopathy (continuous infusion)
question
Fluorouracil 5-FU
answer
Pharmacogenmoics DPD Dihydropyrimidine Dehydrogenase is response for catabolism of 5-FU to inactive metabolites 3% of pts estimated to be heterozygous for the mutant DYPD gene which results in complete or almost complete deficiency of DPD May result in severe toxicity and even death after 5-FU admin in this population
question
Why leucovorin with FU?
answer
Leucovorin is metabolized to methylene-tetrahydrofolate which enhances 5-FU binding to cellular thymidilate synthetase resulting in greater intensity and duration of tumor cytotoxicity
question
Capecitabine
answer
Orally active prodrug of 5-FU that undergoes 3 step conversion to 5-FU w/ final step of phosphorylation by thymidine phosphorylase TP within the cell. TP is higher in tumor cells which results in more selective tumor cytotoxicity Toxicities Dose limiting - diarrhea and hand foot syndrome Less common - N/V (taken after meals), fatigue, myelosuppresion and rash
question
Oxaliplatin
answer
related to platinum analogs that bind to N-7 position on guanine Selective efficacy may be tied to increased expression of MMR genes in colorectal cancer Toxicities minimal nephrotoxicity and ototoxicity less emetogenic than cisplatin
question
Oxaliplatin toxicities
answer
Toxicities minimal nephrotoxicity and ototoxicity less emetogenic than cisplatin Dose limiting toxicities - 2 forms of cumulative peripheral neuropathy 1) acute - within 1st 2 days of infusion -exacerbated by cold -mostly peripheral extremities (fingers, toes, palms, soles) -reversible (usually within 14 days) 2) delayed usually presents more than 14 days post infusion persistent may interfere with writing, buttoning, swallowing most common reason for discontinuation which may result in some improvement
question
Irinotecan
answer
Topo I inhibitors Active metabolite is SN38 SN38 is glucuronidated to inactive metabolites by UDP-glucuronosyltransferase UGT1A1 reduced or deficient UGT1A1 levels occur in Gilbert's familial hyperbilirubinemia syndrome May result in severe irinotecan-induced diarrhea and/or neutropenia Should be tested for in patients with elevated bilirubin and or persistent toxicity and result in an irinotecan dose reduction if positive
question
Irinotecan Toxicities
answer
Dose limiting - 2 forms of cumulative diarrhea 1) early onset during infusion within 24 hours 8% incidence cholinergic in nature and includes flushing, lacrimation prevented and treated with atropine 0.25-1mg IV 2) late onset 10-14 days post infusion 31% incidence may be reduced with intensive loperamide therapy - 4 mg at earliest sign then 2 mg q1-2 hours until diarrhea free for 12 hours. Other agents include diphenoxylate/atropine, octreotide and tincture of opium Other Toxicities = neutropenia, nausea, vomiting
question
Angiogensis
answer
Tumor have blood vessels so new therapies can prevent angiogenesis
question
Bevacizumab
answer
MOA 1st angiogenesis inhibitor to demonstrate efficacy in treating solid tumors Approved 1st line therapy with 5-FU based chemo for metastatic colorectal cancers
question
Bevacizumab toxicities
answer
Dose limiting toxicities Hypertension (monitor before infusion) Bleeding episodes VTEs - MI, PE, DVT Other toxicities Impaired wound healing, bowel perforation, proteinuria
question
Bevacizumab Precautions
answer
Stop bevacizumab at least 4 weeks prior to surgery Do not initiate or re-start for at least 4 weeks post surgery greater risk of VTE patients 65 yo+ 2009 UWMC/SCCA study clinical significant proteinuria is rare (1.2%) and routine proteinuria monitoring is associated with high costs
question
Cetuximab (Erbitux) Panitumumab (Vectibix) MOA ***Not interchangable after treatment failure***
answer
Both agents bind to cell surface epidermal growth factor receptor EGFR-1 inhibiting EGF and TGF-alpha binding and signal transduction Indicated now in combo w/ 5-FU based regs or as single agents for refractory disease only for metastatic colorectal cancers expressing the KRAS, NRAS wild type WT gene Not effective in tumors expressing the KRAS gene mutation at codons 12 and 13 (EGFR testing is no longer recommended) Cetuximab should not be used after treatment failure on panitumumab Panitumumab should not be used after treatment failure on cetuximab
question
Cetuximab (Erbitux) Panitumumab (Vectibix) Toxicities
answer
2 kinds of rash in patients that don't have mutations Infusion related reactions 1% with panitumumab - fully human MAB Acne-from skin rash - common and indicator of tumor response prevention and treatment of rash includes oral minocycline or doxycycline, clindamycin gel, corticosteroid cream and oral corticosteroids for more severe cases less common toxicities NVD interstitial lung disease
question
Regorafenib MOA Dosing
answer
multi-kinase/angiogenesis inhibitor indicated for 4th line therapy in metastatic colorectal cancer patients in 3rd progression previously treated with FOLFOX or CAPOX, FOLFIRI, bevacizumab and cetuximab or panitumumab if KRAS wild type Dosing - 160mg PO daily days 1-21 of 28 day cycle
question
Regorafenib Toxicities
answer
Toxicities (may be dosing limiting) Dysphonia hand foot syndrome hepatoxocities hemorrhage HTN Cardiac ischemia and infarction RPLS GI perforation or fistula impaired wound healing fatigue acne-form rash headache mucositis diarrhea pain fever asthenia electrolyte disorders myelosuppression proteinuria increased INR
question
Regorafenib Concerns
answer
CYP3A4 interactions Special low fat breakfast instructions Open bottle of 28 tablets expires in 28 days $10,000 per month
question
Trifluidine/Tipiracil = LONSURF MOA/Dosing
answer
combo trifluridine = nucleoside metabolic inhibitor tipiracil = thymidine phosphorylase inhibitor indicated for 4th line therapy in metastatic colorectal cancer patients in 3rd progression previously treated with FOLFOX or CAPOX, FOLFIRI, bevacizumab, cetuximab or panitumumab if KRAS wild type Dosing - based on trifluridine 35 mg/m2 dose BID on days 1-5 and 8-12 of each 28 day cyle
question
Trifluidine/Tipiracil = LONSURF Toxicities
answer
may be dose limiting neutropenia thrombocytopenia anemia fatigue acne-form rash headache mucositis N/D abdominal pain fever dysgeusia allopecia
question
Trifluidine/tipiracil Concerns
answer
RCT found difference in OS of 1.8 months compared to placebo Round doses to nearest 5 mg Must be taken within 1 hour of meal $12,000 for 28 day supply



