Introduction to Organic Chemistry – Flashcards
Unlock all answers in this set
Unlock answersquestion
Organic Compounds
answer
-Organic Compounds is the study of carbon-containing compounds -Carbon can bond to other carbons forming many compounds. -Organic compounds contain C-C and C-H covalent bonds. -Some organic compounds have *heteroatoms*.
question
Heteroatoms
answer
-(O, N, P, S, and halogens) Halogens: F,Cl,Br,I
question
Forumula of an organic compound is written as
answer
-CaHbXd X = C, N, P, S, or halogen
question
General Properties of Oragnic Compounds
answer
-Most are nonpolar covalent compounds. -Weak interactions (dispersion forces) between molecules. Have low melting points and boiling points. -They are flammable. Undergo Combustion. -Most are not soluble in water - they are hydrophobic (water fearing) -Polar compounds (when polar groups present, Ex: OH group) are soluble in water - hydrophillic (water loving) -Less Dense than Water
question
Hydrocarbons
answer
are organic compounds that contrain only carbon and hydrogen.
question
Types of Hydrocarbons
answer
1. Alkanes 2. Alkenes 3. Alkynes 4. Aromatic Hydrocarbons
question
Alkanes
answer
-Carbons joined by single bonds only. -They are saturated hydrocarbons because they have the maximum number of possible hydrogens. -2n + 2 no. of hydrogens, where n is the no. of carbons -Carbon has four valence electrons; hydrogen has one. Each carbon forms four bonds to achieve an octet. Ex: CH4
question
Alkenes
answer
-Contrain one or more Carbon-Carbon double bonds. -Unsataturated hydrocarbons because they have less than the maximum no. of H. (for each double bond, 2 less hydrogens) -The general formula of a alkene with one double bond is C8H2n -Simplest Alkene is: C2H4 -Carbons with double bonds have trigonal planar molecular geometries with 120 degree bond angles.
question
Alkynes
answer
-Contain one or more Carbon-Carbon triple bonds. -Unsaturated. For each triple bond, 4 less hydrogen. -Forumla for an alkyne with one triple bond is CnH2n-2 -Simpliest Alkyne os: C2H2 -Triple bonded carbons have linear molecular geometries with 18- degree bond angles.
question
Aromatic Hydrocarbons
answer
-Ring structures with alternating double and sinlge bonds. -Unsaturated. -Simplest aromatic Hydrocarbon is benzene. -Benzene has 6 Carbon atoms arranged in a shape of a hexagon -One Hydrogen atom is attached to each carbon. -There are two possible structions, which are called resonance structures.
question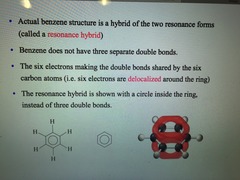
Benzene Structure

answer
-Actual Benzene Structure is a hybrid of the two resonance forms (called a resonance hybrid) -Benzene does not have three separate double bonds. -The six electrons making the double bongs shared by the six carbon atoms (i.e. six electrons are delocalized around the ring) -The resonance hybrid is shown with a circle inside the ring, instead of three double bonds.
question
Molecular formulas
answer
~give the total number of each kind of atoms. - Do not show how atoms are connected. Ex: C4H10
question
Molecular models
answer
-show how atoms are connected.
question
Space-filling model
answer
-spheres show relative size and distance between atoms - most accurate way of showing a molecule
question
Ball-and-stick model
answer
- atoms are shown as balls and bonds as sticks
question
Expanded structural formulas
answer
Each atom and bond is explicitly shown.
question
Condensed structural formulas
answer
- show each carbon atom and its hydrogen atoms as a group.
question
Skeletal line structures (skeletal formulas)
answer
- Carbons and hydrogens are omitted. ~Carbon - carbon bonds are shown as lines
question
Number of bonds
answer
H C NP OS F,Cl,Br,I
question
H C NP OS F,Cl,Br,I
answer
H - 1 Bond C - 4 Bonds NP - 3 Bonds OS - 2 Bonds F,Cl,Br,I (Halogens) - 1 Bond



