Epidemiology Lecture 10 – Effect Modification – Flashcards
Unlock all answers in this set
Unlock answersquestion
Effect modification = Interaction Definition: Size of an association differs by a third factor (third factor alters size/amt of correlation between exposure/outcome) - Exposure-outcome relationship differs among subgroups OR - Two or more exposures act together to influence the outcome

answer
Effect Modification
question
Randomized trial: 17,802 people, 26 countries Confounding = Everything! Even unmeasured things --> Confounding is NOT a factor in a randomized study of this size AS LONG AS you analyze data via Intent to Treat groups (placebo vs treatment, not separating into new groups) *Table 1* *Findings*: Incidence proportion CV events - rosuvastatin= 1.6% Incidence proportion CV events - placebo = 2.8% Relative risk = 1.6%/2.8% = 0.57 "Rosuvastatin reduces the risk of cardiovascular events by 43%"
<img src="https://chmanchacentro.com/wp-content/uploads/2018/04/effect-modification-example-clinical-trial-of-rosuvastatin-to-prevent-cardiovascular-events-17802-individuals-with-ldlc-2-0-mg-l-men-e289a550-and-women-e289a560-years-old-excluded-for.jpg" title="Effect Modification Example: Clinical trial of rosuvastatin to prevent cardiovascular events - 17,802 individuals with LDLc 2.0 mg/L - Men ≥50 and women ≥60 years old - Excluded for prevalent cardiovascular disease - Randomly assigned to either rosuvastatin 20 mg per day or placebo - Primary outcome was first major CV event (MI, stroke, unstable angina, revascularization, or cardiovascular death)" alt="Effect Modification Example: Clinical trial of rosuvastatin to prevent cardiovascular events - 17,802 individuals with LDLc 2.0 mg/L - Men ≥50 and women ≥60 years old - Excluded for prevalent cardiovascular disease - Randomly assigned to either rosuvastatin 20 mg per day or placebo - Primary outcome was first major CV event (MI, stroke, unstable angina, revascularization, or cardiovascular death)">
answer
Effect Modification Example: Clinical trial of rosuvastatin to prevent cardiovascular events - 17,802 individuals with LDLc 2.0 mg/L - Men ≥50 and women ≥60 years old - Excluded for prevalent cardiovascular disease - Randomly assigned to either rosuvastatin 20 mg per day or placebo - Primary outcome was first major CV event (MI, stroke, unstable angina, revascularization, or cardiovascular death)
question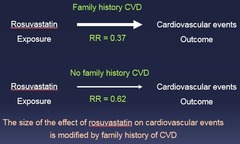
The size of the effect of rosuvastatin on cardiovascular events is modified by family history of CVD

answer
Effect Modification Example: A third factor is influencing the outcome!
question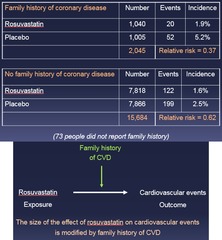
Family history of CVD stratum Relative risk = 0.37 No family history of CVD stratum Relative risk = 0.62 *These relative risks are different from each other* --> Data suggest that the effect of rosuvastatin on CV events differs between people w/ and w/out a family history of CVD = *A third factor (Family Hx) is influencing the outcome!*

answer
Effect Modification Example Stratification:
question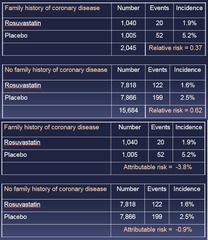
Relative or Attributable Risk - Both risks demonstrate effect modification by Fam Hx of CVD

answer
The size of the effect of rosuvastatin on cardiovascular events is modified by family history of CVD: Example
question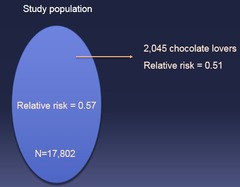
When pulling a subgroup out of a population by random, you may get sligh deviations from "truth" in results due to chance characteristics of the group of individuals chosen (e.g. you might happen to pick more chocolate lovers by pure chance)

answer
Effect Modification by Chance
question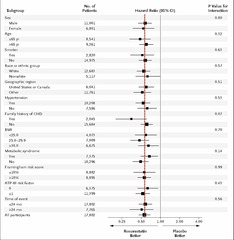
Plot of relative risks by subgroups - bottom = RR for entire study population - can look for particularly strong relationships

answer
Forest Plot
question
- Biologic plausibility - Subgroup specified prior to analysis - Large numbers of outcomes in subgroups Statistical Considerations: - Passes statistical test for interaction - Non-overlapping confidence intervals
answer
When to get (a little bit) excited about different effects in subgroups
question
*When they are replicated in independent populations* = GOLD STANDARD - Subgroup analyses often referred to as "hypothesis generating" analyses
answer
When to get very excited about different effects in subgroups
question
*Estimate the likely range of effects in the population*

answer
Confidence Intervals
question
1.) Among all similar people who have a fam hx of CVD - RR of CV events comparing rosuvastatin to placebo is probably somewhere roughly between 0.11-0.60 2) Among all similar people who do not have a fam hx of CVD - RR of CV events comparing rosuvastatin to placebo is probably somewhere roughly between 0.44-0.72 RRs different from each other in this study Based on these (non???) overlapping confidence intervals there is uncertainty as to whether the relative risks are actually different in the population

answer
Confidence Intervals: Example
question
Estimates the chance of observing this difference in effect size if the effect sizes were in fact equal in the population
answer
P-for Interaction
question
Given no difference in these RR's among the entire populations of people with and without a family history of CVD, the chance of observing these different relative risks in this study is 7% - A low p-for-interaction value (<0.05) infers that the observed subgroup differences likely to be true in the population

answer
P-for Interaction: Example
question
Pic
answer
Effect Modification Example 2: New example: clinical trial of trastuzumab, a monoclonal antibody against Human Epidermal Growth Factor Receptor type 2 (HER2) Randomized trial in women who have lymph node+ breast cancer: 1.) Doxirubicin + cyclophosphamide + trastuzumab 2.) Doxirubicin + cyclophosphamide + placebo Pre-specified plan to assess whether anti-cancer effects of trastuzumab are stronger in women who have HER2+ breast cancer
question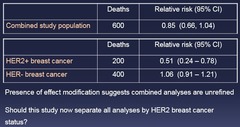
Presence of effect modification suggests combined analyses are unrefined Should this study now separate all analyses by HER2 breast cancer status? - Putting it together might be hiding something but it depends on what the item is - subjective calll

answer
Effect Modification Example 2 - Stratification or No?
question
Big randomized trial as in this case = no confounding = Effect Modifier Small observational finding = might be association between HER2 status and breast cancer status
answer
Is HER2 Breast Cancer a Confounder or an Effect Modifier?
question
2x2 Table of Exposure vs Exposure showing incidence proportion of Outcome: 1.) Among individuals who do not have HF: 4% extra contrast nephropathy cases associated w/ diabetes 2.) Among individuals who do not have DM : 3% extra contrast nephropathy cases associated w/ HF 3.) EXPECT 4%+3% = 7% extra contrast nephropathy cases associated w/ BOTH DM and HF 4.) OBSERVE 30% extra contrast nephropathy cases associated w/ BOTH DM and HF = More than expected = Synergistic relationship --> *In this example, study data suggest interaction BETWEEN DM and HF on the risk of contrast nephropathy* The two exposures are synergizing and the *effect is more than the sum of their parts*
answer
Effect Modification Example 3 *Synergy*: Diabetes, heart failure, and contrast nephropathy - Cohort study of 15,400 people undergoing an IV contrast study - Diabetes and heart failure assessed at baseline - Contrast nephropathy defined as ≥25% increase in serum creatinine levels within 48 hours of contrast administration Q is not whether DM or HF contribute to contrast nephropathy but rather, do the 2 work together?
question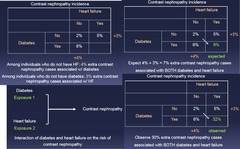
Evaluate via 2x2 Table of Exposure vs Exposure showing incidence proportion of Outcome - Compare expected vs Observed - If Observed > Synergistic Expected = Effect Modification The two exposures are synergizing and the *effect is more than the sum of their parts*

answer
Effect Modification Synergy
question
The two exposures are synergizing and the *effect is more than the sum of their parts*
*In this example, study data suggest interaction BETWEEN DM and HF on the risk of contrast nephropathy*" alt="Effect Modification Example 3 *Synergy*: EXPECT 4%+3% = 7% extra contrast nephropathy cases associated w/ BOTH DM and HF OBSERVE 30% extra contrast nephropathy cases associated w/ BOTH DM and HF = More than expected = Synergistic relationship --> *In this example, study data suggest interaction BETWEEN DM and HF on the risk of contrast nephropathy*">
answer
Effect Modification Example 3 *Synergy*: EXPECT 4%+3% = 7% extra contrast nephropathy cases associated w/ BOTH DM and HF OBSERVE 30% extra contrast nephropathy cases associated w/ BOTH DM and HF = More than expected = Synergistic relationship --> *In this example, study data suggest interaction BETWEEN DM and HF on the risk of contrast nephropathy*
question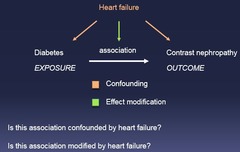
1.) Is the association confounding? 2.) Is the association modified by X?

answer
Effect Modification vs Confounding: Two Questions to Ask
question
1.) First step is to determine the primary exposure - outcome association - Relative Risk(s) 2.) Is this association confounded by (factor)? - Adjust risk and compare adjusted vs non-adjusted 2.) Is this association modified by (factor)? - Do the weighed and combined risks differ from each other? - If YES, then there is modification
answer
Effect Modification vs Confounding
question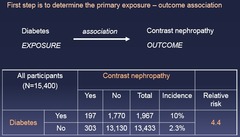
Determine the primary exposure-outcome association - e.g. Diabetes associated with 4.4-fold greater risk of contrast nephropathy *Relative Risk*

answer
Effect Modification vs Confounding: Step 1
question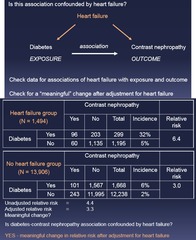
Is this association confounded by (factor)? 1.) Relative Risk 2.) Adjust for HF and compare adjusted vs non-adjusted

answer
Effect Modification vs Confounding: Step 2
question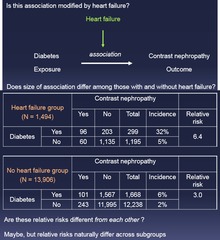
Is this association modified by (factor)? 1.) Do the weighed and combined risks differ from each other? - If YES, then there is modification

answer
Effect Modification vs Confounding: Step 3
question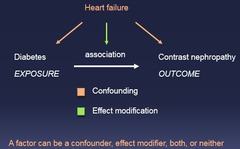
*e.) all of the above* A factor can be a confounder, effect modifier, both, or neither

answer
A factor can be which of the following? a.) a confounder b.) an effect modifier c.) both d.) neither e.) all of the above
question
An exposure-outcome relationship may differ among subgroups: - Subgroup differences may occur due to natural variation - Set of criteria to help decide whether subgroup finding likely valid - Forest plot one method of presenting subgroup differences
answer
Effect Modification via Subgroup-Analysis Summary
question
In stratified analyses: - Confounding suggested if the weighted (adjusted) association is "meaningfully" different from the unadjusted association - Effect modification suggested if the size of the association differs between strata
answer
Effect Modification via Stratified -Analysis Summary



