Chemistry Midterm Study Guide 2016
Unlock all answers in this set
Unlock answersquestion
Mass
answer
the amount of matter in an object
question
Weight
answer
the gravitational pull on an object
question
Scientific method
answer
the process of studying natural phenomena, involving observations, forming laws and theories, and testing of theories by experimentation
question
Scientific method steps
answer
1. identify the problem 2. form a hypothesis 3. create an experiment 4. perform the experiment 5. analyze the experiment 6. communicate the results
question
Qualitative data
answer
data that is observed and not measured; descriptions
question
Quantitative data
answer
data that is measured; numeric
question
Dependent variable
answer
the variable that is measured in the experiment and affected by the experiment
question
Independent variable
answer
the variable that is changed by the scientists, experiments only have 1 of these
question
Mass
answer
Kilogram (Kg)
question
Volume
answer
Liter (L)
question
Length
answer
meter (m)
question
Temperature
answer
Kelvin (k)
question
Electric Current
answer
Ampere (A)
question
Amount of substance
answer
mole (mol)
question
Time
answer
second (s)
question
Temperature Conversions
answer
C= K-273 K= C+273 C= F-32/1.8 F= (C*1.8) +32
question
Density
answer
mass/volume
question
Accuracy
answer
getting as close to the true value as possible
question
Precision
answer
getting the same answer over and over again
question
Random era
answer
errors are random
question
Systematic era
answer
something wrong with the system you are using, low accuracy and high precision
question
Percent error
answer
Actual-experimental/actual x 100
question
Significant figures
answer
-all digits between 1-9 -zeros between significant figures -trailing zeros if the number has a decimal point -zeros following a decimal sig fig
question
Not significant figures
answer
-zeros in the beginning of a number whose only function is to place the decimal point
question
Multiplying/dividing significant figures
answer
limit and round to the least # of sig figs in any of the factors
question
Adding/subtracting significant figures
answer
limit and round to the least # of decimal places in any of the numbers
question
Intensive properties
answer
-do not depend on the amount of matter present -Includes: boiling point freezing point melting point density flammability combustibility corrosiveness condensation point hardness color
question
Extensive properties
answer
-depend on the amount of matter present -Includes: volume mass length weight
question
Physical properties
answer
-properties that are measurable -Includes: color odor texture density volume states of matter melting point boiling point
question
Chemical properties
answer
-material's properties that become evident during/after a chemical reaction -Includes: burning oxidation tarnishing rust fermentation :sugar to alcohol combustion: burning electrolysis: water to hydrogen and oxygen gas
question
Physical change
answer
-change in state of matter -Includes: melting boiling evaporating sublimation: dry ice melting from solid to gas ductile: drawn into a wire malleable: pounded into sheets
question
Chemical change
answer
-changes into a new substance
question
Evidence of chemical change
answer
1. gas produced 2. heat/light is released or absorbed 3. permanent color change 4. formation of precipitation
question
Endothermic
answer
light/heat is absorbed
question
Exothermic
answer
light/heat is released
question
Gas
answer
no definite shape or volume
question
Liquid
answer
definite volume but no definite shape
question
Solid
answer
definite volume and shape
question
Mixture
answer
-blend of 2 or more substances -can be physically separated -not chemically bonded
question
Pure substance
answer
-elements and compounds -requires a chemical process to separate
question
Element
answer
a substance that cannot be broken down into simpler substances by chemical or physical means
question
Compound
answer
a substance with constant composition that can be broken down into elements by chemical composition
question
Homogeneous mixture
answer
mixture that is uniform throughout; solution
question
Heterogeneous mixture
answer
mixture that is not uniform throughout; can see different parts Examples: milk, fog, jello
question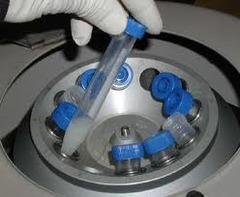
Centrifugation

answer
-process of separating a mixture based on the densities of the particles in that mixture -physical seperation -Example: blood: more dense on bottom, less dense on top
question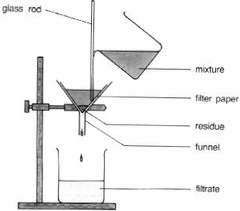
Filtration by Gravity

answer
-process of separating a mixture based on the size of the particles in that mixture -separate an insoluble solid in a liquid -physical separation -Example: strainer
question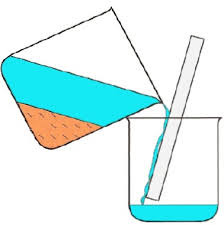
Decantation

answer
-pouring off of a liquid from a mixture that contains both a liquid and a solid -separate 2 immiscible liquids -physical separation -Example: oil and vinegar, oil and water
question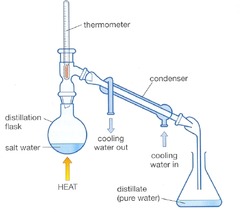
Simple Distillation

answer
-separate a soluble solid in a liquid -physical -Example: salt water
question
Chromatography
answer
-separate miscible liquids -2 phases: mobile and stationery -physical separation Example: separating ink from paper
question
Evaporation

answer
-recover a soluble solid from a liquid -physical separation -Example: salt water, sugar water
question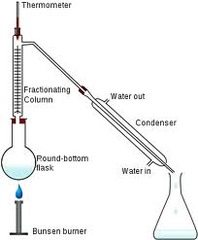
Fractional distillation

answer
-separate 2 or more miscible liquids by their boiling points -physical separation -Example: water and alcohol
question
Seperatory Funnel

answer
-separating 2 immiscible liquids -physical separation -example: oil and vinegar
question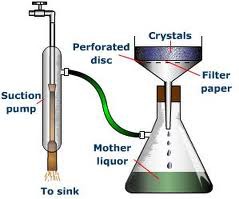
Filtration by vacuum

answer
-collect an insoluble solid from a mixture -physical seperation -Example: sand and water
question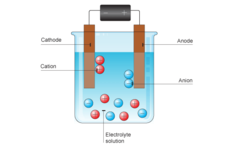
Electrolysis

answer
-separation of hydrogen atoms from the oxygen atoms in a water molecule -chemical separation -Final result: oxygen and hydrogen gas separated
question
Law of conservation of mass
answer
matter cannot be created or destroyed; it can only be converted from 1 form to another; mass of reactants= mass of products
question
Law of definite proportion
answer
-proportion of mass of elements in a compound is always the same -discovered by Proust
question
Law of multiple proportion
answer
-mass of 1 element can combine with fixed masses of another element to make different things -discovered by John Dalton
question
Democritus
answer
-Greek philosopher -among first to suggest existence of atoms
question
Dalton
answer
-used Lavoisier's idea of experimenting -law of multiple proportions -1st atomic theory
question
Dalton's 1st atomic theory
answer
-all matter is made up of atoms -all atoms are indivisible (wrong b/c of subatomic particles-p, n, e) -atoms of different elements are different -atoms of the same elements are the same (wrong b/c of isotopes) -atoms can combine, rearrange, and separate to form new compounds
question
Thomson
answer
-Cathode ray experiment (electrons) -plum pudding model
question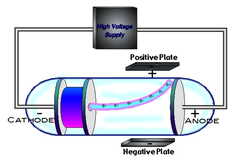
Cathode ray experiment

answer
-discovered electrons -ray negatively charged -ray deflected away from negative, towards positive
question
Cathode ray
answer
stream of electrons produced at the negative electrode of a tube containing a gas at low pressure
question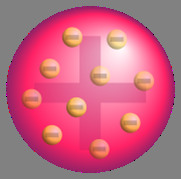
Plum pudding model

answer
negative embedded in positive
question
Ernest Rutherford
answer
-Gold Foil experiment(nucleus and protons)
question
Gold foil experiment
answer
-alpha particles have positive charge and are the size of Helium atoms -expected all particles to go straight through, some were deflected straight back Found: -nucleus is small, dense and contains protons (positive) -discovery of nucleus and protons
question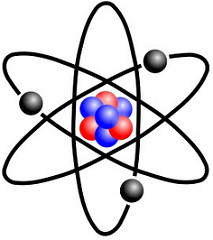
Nuclear Model

answer
-positive nucleus with electrons circling -atom is mostly empty space
question
Mass number
answer
# of protons + neutrons
question
Neutron
answer
-neutral charge -located in the nucleus -atomic mass - atomic #
question
Proton
answer
-positive charge -located in the nucleus -atomic #
question
Electron
answer
-negative charge -located outside the nucleus -atomic # in a neutral atom
question
atomic mass
answer
weighted average mass
question
atomic mass unit (amu)
answer
1/12 the mass of a carbon-12 atom
question
Ion
answer
a charged atom
question
Cation
answer
-positive charge -lose electrons
question
Anion
answer
-negative charge -add electrons
question
Isotopes
answer
atom of the same element with different numbers of neutrons, have a different atomic mass
question
Electron scanning microscope
answer
instrument used to generate images of individual atoms
question
Radioactivity
answer
the spontaneous decomposition of a nucleus to form a different nucleus
question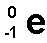
Electron Capture
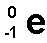
answer
(on left side)
question
Electromagnetic spectrum
answer
all the different forms of electromagnetic radiation, have different wavelengths and frequencies
question
Wavelength
answer
the distance between 2 crests
question
Frequency
answer
numbers of waves that pass
question
Speed of light
answer
frequency x wavelength
question
Energy
answer
E=hv
question
Photoelectric effect
answer
electrons are emitted from a metal surface when light of a certain frequency shines on it
question
Quantum
answer
minimum amount of energy that can be gained or lost by an atom
question
Excited state- ground state
answer
releases energy in form of light: colors produced
question
Bohr quantum model
answer
electrons in specific orbitals (set path), only worked for hydrogen because it has 1 electron
question
Heisenberg uncertainty principle
answer
you cannot know the velocity and position of an electron at the same time, impossible to assign set paths to an electron
question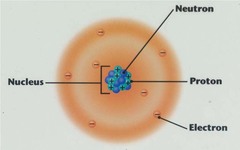
Electron cloud

answer
-discovered by DeBroglie, Schrodinger, Heisenberg, and Einstein
question
S orbital
answer
spherical appears in 1-7 can hold 2 electrons
question
P orbital
answer
dumb-bell appears in 2-7 can hold 6 electrons
question
D orbital
answer
2 dumb-bells appears in 3-7 can hold 10 electrons
question
F orbital
answer
shape too complicated appears in 4-7 can hold 14 electrons
question
Balmer series
answer
visible light
question
Lyman series
answer
UV light
question
Paschen series
answer
Infared light
question
Aufbau principle
answer
electrons must occupy the lowest energy level lst
question
Hund's rule
answer
electrons want to be alone before they pair up
question
Pauli exclusion principle
answer
2 electrons can occupy each platform, must have different spins



